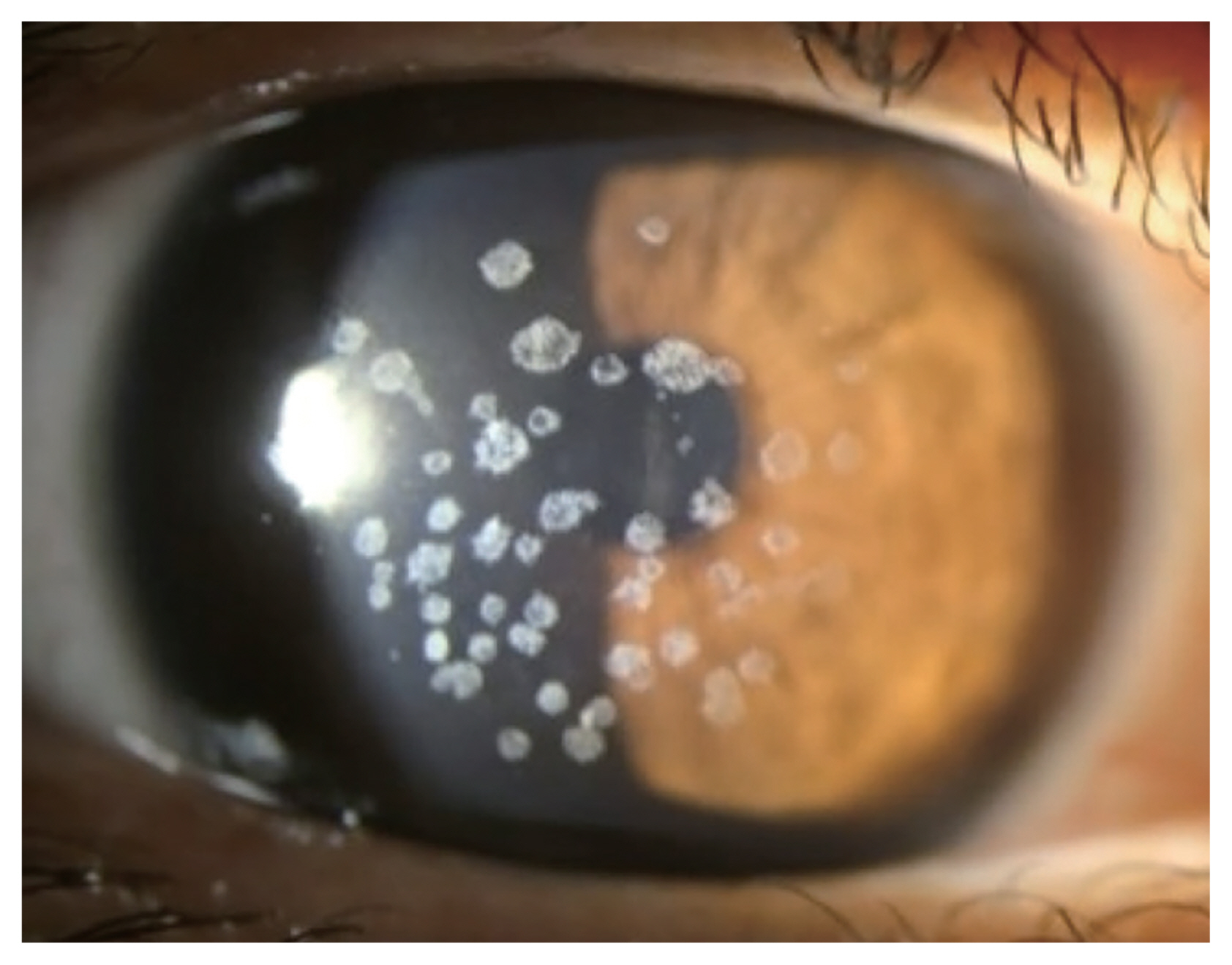
1. Aggarwal S, Peck T, Golen J, Karcioglu ZA. Macular corneal dystrophy: a review.
Surv Ophthalmol 2018;63:609-17.


2. Weiss JS, Moller HU, Aldave AJ, et al. IC3D classification of corneal dystrophies: edition 2.
Cornea 2015;34:117-59.


3. Weiss JS, Moller HU, Lisch W, et al. The IC3D classification of the corneal dystrophies.
Cornea 2008;27(Suppl 2):S1-83.



4. Han KE, Kim TI, Chung WS, et al. Clinical findings and treatments of granular corneal dystrophy type 2 (avellino corneal dystrophy): a review of the literature.
Eye Contact Lens 2010;36:296-9.


5. Holland EJ, Daya SM, Stone EM, et al. Avellino corneal dystrophy. Clinical manifestations and natural history.
Ophthalmology 1992;99:1564-8.


6. Munier FL, Korvatska E, Djemai A, et al. Kerato-epithelin mutations in four 5q31-linked corneal dystrophies.
Nat Genet 1997;15:247-51.



7. Korvatska E, Munier FL, Djemai A, et al. Mutation hot spots in 5q31-linked corneal dystrophies.
Am J Hum Genet 1998;62:320-4.



8. Streeten BW, Qi Y, Klintworth GK, et al. Immunolocalization of beta ig-h3 protein in 5q31-linked corneal dystrophies and normal corneas.
Arch Ophthalmol 1999;117:67-75.


9. Dighiero P, Drunat S, DâHermies F, et al. A novel variant of granular corneal dystrophy caused by association of 2 mutations in the TGFBI gene-R124L and DeltaT125-DeltaE126.
Arch Ophthalmol 2000;118:814-8.


10. Sakimoto T, Kanno H, Shoji J, et al. A novel nonsense mutation with a compound heterozygous mutation in TGFBI gene in lattice corneal dystrophy type I.
Jpn J Ophthalmol 2003;47:13-7.


11. Moon JW, Kim SW, Kim TI, et al. Homozygous granular corneal dystrophy type II (Avellino corneal dystrophy): natural history and progression after treatment.
Cornea 2007;26:1095-100.

12. Lisch W, Weiss JS. Clinical and genetic update of corneal dystrophies.
Exp Eye Res 2019;186:107715.


13. Lee JH, Cristol SM, Kim WC, et al. Prevalence of granular corneal dystrophy type 2 (Avellino corneal dystrophy) in the Korean population.
Ophthalmic Epidemiol 2010;17:160-5.


14. Afshari NA, Mullally JE, Afshari MA, et al. Survey of patients with granular, lattice, avellino, and Reis-BĂźcklers corneal dystrophies for mutations in the BIGH3 and gelsolin genes.
Arch Ophthalmol 2001;119:16-22.

15. Nowinska AK, Wylegala E, Janiszewska DA, et al. Genotype-phenotype correlation of TGFBI corneal dystrophies in Polish patients.
Mol Vis 2011;17:2333-42.


16. Han KE, Choi SI, Kim TI, et al. Pathogenesis and treatments of TGFBI corneal dystrophies.
Prog Retin Eye Res 2016;50:67-88.


17. Choi SI, Kim BY, Dadakhujaev S, et al. Impaired autophagy and delayed autophagic clearance of transforming growth factor β-induced protein (TGFBI) in granular corneal dystrophy type 2.
Autophagy 2012;8:1782-97.



18. Han KE, Chung WS, Kim T, et al. Changes of clinical manifestation of granular corneal deposits because of recurrent corneal erosion in granular corneal dystrophy types 1 and 2.
Cornea 2013;32:e113-20.


19. Folberg R, Alfonso E, Croxatto JO, et al. Clinically atypical granular corneal dystrophy with pathologic features of lattice-like amyloid deposits. A study of these families.
Ophthalmology 1988;95:46-51.


20. Kim TI, Kim H, Lee DJ, et al. Altered mitochondrial function in type 2 granular corneal dystrophy.
Am J Pathol 2011;179:684-92.



21. Aldave AJ, Gutmark JG, Yellore VS, et al. Lattice corneal dystrophy associated with the Ala546Asp and Pro551Gln missense changes in the TGFBI gene.
Am J Ophthalmol 2004;138:772-81.


22. Klintworth GK, Bao W, Afshari NA. Two mutations in the TGFBI (BIGH3) gene associated with lattice corneal dystrophy in an extensively studied family.
Invest Ophthalmol Vis Sci 2004;45:1382-8.


23. Frising M, Wildhardt G, Frisch L, Pitz S. Recurrent granular dystrophy of the cornea: an unusual case.
Cornea 2006;25:614-7.

24. Yamada N, Kawamoto K, Morishige N, et al. Double mutation (R124H, N544S) of TGFBI in two sisters with combined expression of Avellino and lattice corneal dystrophies.
Mol Vis 2009;15:974-9.


25. Zhong X, Chen S, Huang W, et al. Novel and known mutations of TGFBI, their genotype-phenotype correlation and structural modeling in 3 Chinese families with lattice corneal dystrophy.
Mol Vis 2010;16:224-30.


26. Niel-Butschi F, Kantelip B, Iwaszkiewicz J, et al. Genotype-phenotype correlations of TGFBI p.Leu509Pro, p. Leu509Arg, p.Val613Gly, and the allelic association of p.Met502Val-p.Arg555Gln mutations.
Mol Vis 2011;17:1192-202.


27. Yam GH, Wang K, Jhanji V, et al. In vitro amyloid aggregate forming ability of TGFBI mutants that cause corneal dystrophies.
Invest Ophthalmol Vis Sci 2012;53:5890-8.


28. Song JS, Lim DH, Chung ES, et al. Mutation analysis of the TGFBI gene in consecutive Korean patients with corneal dystrophies.
Ann Lab Med 2015;35:336-40.



29. Chae H, Kim M, Kim Y, et al. Mutational spectrum of Korean patients with corneal dystrophy.
Clin Genet 2016;89:678-89.


30. Ann LB, Abbouda A, Frausto RF, et al. Variant lattice corneal dystrophy associated with compound heterozygous mutations in the
TGFBI gene.
Br J Ophthalmol 2017;101:509-13.


32. Lee JH, Chung SH, Stulting RD, et al. Effects of corneal neovascularization on the manifestations of Avellino corneal dystrophy (granular corneal dystrophy type II).
Cornea 2006;25:914-8.


33. Roh MI, Chung SH, Stulting RD, et al. Preserved peripheral corneal clarity after surgical trauma in patients with Avellino corneal dystrophy.
Cornea 2006;25:497-8.


34. Mashima Y, Konishi M, Nakamura Y, et al. Severe form of juvenile corneal stromal dystrophy with homozygous R124H mutation in the keratoepithelin gene in five Japanese patients.
Br J Ophthalmol 1998;82:1280-4.



35. Okada M, Yamamoto S, Inoue Y, et al. Severe corneal dystrophy phenotype caused by homozygous R124H keratoepithelin mutations.
Invest Ophthalmol Vis Sci 1998;39:1947-53.

36. Watanabe H, Hashida Y, Tsujikawa K, et al. Two patterns of opacity in corneal dystrophy caused by the homozygous BIG-H3 R124H mutation.
Am J Ophthalmol 2001;132:211-6.


37. Tsujikawa K, Tsujikawa M, Watanabe H, et al. Allelic homogeneity in Avellino corneal dystrophy due to a founder effect.
J Hum Genet 2007;52:92-7.



38. Jun RM, Tchah H, Kim TI, et al. Avellino corneal dystrophy after LASIK.
Ophthalmology 2004;111:463-8.


39. Banning CS, Kim WC, Randleman JB, et al. Exacerbation of Avellino corneal dystrophy after LASIK in North America.
Cornea 2006;25:482-4.


40. Roh MI, Grossniklaus HE, Chung SH, et al. Avellino corneal dystrophy exacerbated after LASIK: scanning electron microscopic findings.
Cornea 2006;25:306-11.

41. Aldave AJ, Sonmez B, Forstot SL, et al. A clinical and histopathologic examination of accelerated TGFBIp deposition after LASIK in combined granular-lattice corneal dystrophy.
Am J Ophthalmol 2007;143:416-9.


42. Kim TI, Kim T, Kim SW, Kim EK. Comparison of corneal deposits after LASIK and PRK in eyes with granular corneal dystrophy type II.
J Refract Surg 2008;24:392-5.


43. Lee JH, Stulting RD, Lee DH, et al. Exacerbation of granular corneal dystrophy type II (Avellino corneal dystrophy) after LASEK.
J Refract Surg 2008;24:39-45.


44. Kwak JJ, Yoon SH, Seo KY, et al. Exacerbation of granular corneal dystrophy type 2 after small incision lenticule extraction.
Cornea 2021;40:519-24.



45. Maclean H, Robinson LP, Wechsler AW, Goh A. Excimer phototherapeutic keratectomy for recurrent granular dystrophy.
Aust N Z J Ophthalmol 1996;24:127-30.

46. Kim TI, Hong JP, Ha BJ, et al. Determination of treatment strategies for granular corneal dystrophy type 2 using Fourier-domain optical coherence tomography.
Br J Ophthalmol 2010;94:341-5.


47. Jung SH, Han KE, Stulting RD, et al. Phototherapeutic keratectomy in diffuse stromal haze in granular corneal dystrophy type 2.
Cornea 2013;32:296-300.


48. Starr M, Donnenfeld E, Newton M, et al. Excimer laser phototherapeutic keratectomy.
Cornea 1996;15:557-65.


49. Orndahl MJ, Fagerholm PP. Treatment of corneal dystrophies with phototherapeutic keratectomy.
J Refract Surg 1998;14:129-35.


50. Dinh R, Rapuano CJ, Cohen EJ, Laibson PR. Recurrence of corneal dystrophy after excimer laser phototherapeutic keratectomy.
Ophthalmology 1999;106:1490-7.


51. Dogru M, Katakami C, Nishida T, Yamanaka A. Alteration of the ocular surface with recurrence of granular/avellino corneal dystrophy after phototherapeutic keratectomy: report of five cases and literature review.
Ophthalmology 2001;108:810-7.


52. Chen M, Xie L. Features of recurrence after excimer laser phototherapeutic keratectomy for anterior corneal pathologies in North China.
Ophthalmology 2013;120:1179-85.


53. Reddy JC, Rapuano CJ, Nagra PK, Hammersmith KM. Excimer laser phototherapeutic keratectomy in eyes with corneal stromal dystrophies with and without a corneal graft.
Am J Ophthalmol 2013;155:1111-8.


54. Jun I, Jung JW, Choi YJ, et al. Long-term clinical outcomes of phototherapeutic keratectomy in corneas with granular corneal dystrophy type 2 exacerbated after LASIK.
J Refract Surg 2018;34:132-9.


55. Inoue T, Watanabe H, Yamamoto S, et al. Recurrence of corneal dystrophy resulting from an R124H Big-h3 mutation after phototherapeutic keratectomy.
Cornea 2002;21:570-3.


56. Ha BJ, Kim TI, Choi SI, et al. Mitomycin C does not inhibit exacerbation of granular corneal dystrophy type II induced by refractive surface ablation.
Cornea 2010;29:490-6.


57. Kim TI, Choi SI, Lee HK, et al. Mitomycin C induces apoptosis in cultured corneal fibroblasts derived from type II granular corneal dystrophy corneas.
Mol Vis 2008;14:1222-8.


58. Choi SI, Maeng YS, Kim TI, et al. Lysosomal trafficking of TGFBIp via caveolae-mediated endocytosis.
PLoS One 2015;10:e0119561.



59. Jung SH, Han KE, Sgrignoli B, et al. Intraocular lens power calculations for cataract surgery after phototherapeutic keratectomy in granular corneal dystrophy type 2.
J Refract Surg 2012;28:714-24.


60. Yagi-Yaguchi Y, Negishi K, Saiki M, et al. Comparison of the accuracy of newer intraocular lens power calculation methods in eyes that underwent previous phototherapeutic keratectomy.
J Refract Surg 2019;35:310-6.


61. Yoneyama R, Kamiya K, Iijima K, et al. Predictability of intraocular lens power calculation in eyes after phototherapeutic keratectomy.
Jpn J Ophthalmol 2020;64:62-7.



62. Ellies P, Renard G, Valleix S, et al. Clinical outcome of eight BIGH3-linked corneal dystrophies.
Ophthalmology 2002;109:793-7.


63. Park KA, Ki CS, Chung ES, Chung TY. Deep anterior lamellar keratoplasty in Korean patients with Avellino dystrophy.
Cornea 2007;26:1132-5.


64. Salouti R, Hosseini H, Eghtedari M, Khalili MR. Deep anterior lamellar keratoplasty with melles technique for granular corneal dystrophy.
Cornea 2009;28:140-3.


65. Unal M, Arslan OS, Atalay E, et al. Deep anterior lamellar keratoplasty for the treatment of stromal corneal dystrophies.
Cornea 2013;32:301-5.


66. Sundmacher R, Spelsberg H, Reinhard T. Homologous penetrating central limbokeratoplasty in granular and lattice corneal dystrophy.
Cornea 1999;18:664-70.


67. Dunaief JL, Ng EW, Goldberg MF. Corneal dystrophies of epithelial genesis: the possible therapeutic use of limbal stem cell transplantation.
Arch Ophthalmol 2001;119:120-2.

68. Spelsberg H, Reinhard T, Henke L, et al. Penetrating limbo-keratoplasty for granular and lattice corneal dystrophy: survival of donor limbal stem cells and intermediate-term clinical results.
Ophthalmology 2004;111:1528-33.


69. Lang SJ, Eberwein P, Reinshagen H, et al. Simultaneous transplantation of limbal stem cells may reduce recurrences of granular dystrophy after corneal transplantation: 2 long-term case reports.
Medicine (Baltimore) 2015;94:e789.


70. Mashima Y, Kawai M, Yamada M. Corneal electrolysis for recurrence of corneal stormal dystrophy after keratoplasty.
Br J Ophthalmol 2002;86:273-5.


71. Choi SI, Kim BY, Dadakhujaev S, et al. Inhibition of TGF-BIp expression by lithium: implications for TGFBI-linked corneal dystrophy therapy.
Invest Ophthalmol Vis Sci 2011;52:3293-300.


72. Choi SI, Dadakhujaev S, Ryu H, et al. Melatonin protects against oxidative stress in granular corneal dystrophy type 2 corneal fibroblasts by mechanisms that involve membrane melatonin receptors.
J Pineal Res 2011;51:94-103.


73. Choi SI, Kim KS, Oh JY, et al. Melatonin induces autophagy via an mTOR-dependent pathway and enhances clearance of mutant-TGFBIp.
J Pineal Res 2013;54:361-72.



74. Yuan C, Zins EJ, Clark AF, Huang AJ. Suppression of keratoepithelin and myocilin by small interfering RNAs (siRNA) in vitro.
Mol Vis 2007;13:2083-95.

75. Courtney DG, Atkinson SD, Moore JE, et al. Development of allele-specific gene-silencing siRNAs for TGFBI Arg-124Cys in lattice corneal dystrophy type I.
Invest Ophthalmol Vis Sci 2014;55:977-85.


76. Kim EK, Kim S, Maeng YS. Generation of TGFBI knockout ABCG2+/ABCB5+ double-positive limbal epithelial stem cells by CRISPR/Cas9-mediated genome editing.
PLoS One 2019;14:e0211864.










 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print