In Vitro Effects of Preservative-free and Preserved Prostaglandin Analogs on Primary Cultured Human Conjunctival Fibroblast Cells
Article information
Abstract
Purpose
Long-term use of topical medication is needed for glaucoma treatment. One of the most commonly prescribed classes of hypotensive agents are prostaglandin analogs (PGs) used as both first-line monotherapy; as well as in combination therapy with other hypotensive agents. Several side effects of eye drops can be caused by preservatives. The purpose of this study was to evaluate the effects of PGs with varying concentrations of benzalkonium chloride (BAC), alternative preservatives, or no preservatives on human conjunctival fibroblast cells.
Methods
Primary human conjunctival fibroblast cells were used in these experiments. Cells were exposed to the following drugs: BAC at different concentrations, bimatoprost 0.01% (with BAC 0.02%), latanoprost 0.005% (with BAC 0.02%), tafluprost 0.0015% with/without 0.001% BAC and travoprost 0.004% (with 0.001% Polyquad) for 15 and 30 minutes. Cell cytotoxicity was evaluated by phase-contrast microscopy to monitor morphological changes of cells, Counting Kit-8 (CCK-8) assay to cell viability, and fluorescent activated cell sorting (FACS) analysis to measure apoptosis.
Results
BAC caused cell shrinkage and detachment from the plate in a dose-dependent manner. Morphological changes were observed in cells treated with bimatoprost 0.01% and latanoprost 0.005%. However, mild cell shrinkage was noted in cells treated with tafluprost 0.0015%, while a non-toxic effect was noted with travoprost 0.004% and preservative-free tafluprost 0.0015%. CCK-8 assay and FACS analysis showed all groups had a significantly decreased cell viability and higher apoptosis rate compared with the control group. However, travoprost 0.004% and preservative-free tafluprost 0.0015% showed lower cytotoxicity and apoptosis rate than other drugs.
Conclusions
This in vitro study revealed that BAC-induced cytotoxicity is dose-dependent, although it is important to emphasize that the clinical significance of toxicity differences observed among the different PGs formulations has not yet been firmly established. Alternatively preserved or preservative-free glaucoma medications seem to be a reasonable and viable alternative to those preserved with BAC.
Long-term use of topical medication is needed to treat for glaucoma. Chronic use of topical anti-glaucomatic agents induce side effects, such as ocular inflammation, allergy, dry eye syndrome, and failure of filtration surgery [1-9]. The side effects of eye drops are caused by components such as preservatives. Currently, benzalkonium chloride (BAC) is the most commonly used preservative in ophthalmic preparations. BAC, a quaternary ammonium compound, is a highly effective antimicrobial agent that acts by denaturing proteins and disrupting cytoplasmic membranes [10]. In the eyes, BAC turnover is very slow and is retained in ocular tissues up to 48 hours after administering a single drop [11].
One of the most commonly prescribed classes of hypotensive agents are prostaglandin analogs (PGs), which are used as both first-line monotherapy as well as in combination therapy with other hypotensive agents [3]. PGs are superior to beta-adrenoceptor antagonists in terms of lowering intraocular pressure (IOP), and they have no severe systemic side effects during long-term clinical use [12,13].
New PG formulations without BAC or with alternative preservatives are currently in development. Several studies have revealed that alternative preservatives to BAC, for example sofZia (Alcon, Fort Worth, TX, USA), Purite (a stabilized oxychloro complex), and Polyquad, produce fewer corneal changes and less conjunctival inf lammation than BAC [1-3,14,15]. Preservative-free PGs are currently commercially available, and their low toxicity to the corneal and conjunctival surfaces has been confirmed [4].
The purpose of this study was to evaluate the effects of PGs with varying concentrations of BAC or alternative preservatives on human conjunctival fibroblast cells.
Materials and Methods
Preparation of human tissues and cell culture
Normal human conjunctival tissues were obtained from two patients during cataract surgery. All tissues were removed after obtaining informed consent from the donor. Immediately after excision, the tissue was placed into either Hank's balanced salt solution or phosphate buffered saline (PBS) containing a penicillin (5,000 U/mL)/streptomycin (5,000 µg/mL) mixture (Gibco BRL, Gaithersburg, MD, USA). Tissues were finely minced into 1-mm3 pieces and placed in six-well culture plates containing 1 mL of Dulbecco's modified Eagle medium (DMEM)/F12 (1:1 vol/vol) containing 10% fetal bovine serum (Gibco BRL), ITS (5 mg/mL insulin, 30 nM selenium, 25 mg/mL human transferrin; Sigma, St. Louis, MO, USA), and a penicillin (5,000 U/mL)/streptomycin (5,000 µg/mL) mixture. Seeded tissues were incubated at 37℃ in a humidified air atmosphere with 5% CO2 and fed daily. When cells began to form a monolayer, tissue pieces were removed. When conjunctival fibroblast cultures reached confluence, they were detached from the dishes with trypsin-ethylenediaminetetraacetic acid and replated into new dishes at a ratio of 1:3. Primary human conjunctival fibroblast cells (passages four and five) were used in these experiments.
Drug treatment
After dividing the cells (6 × 103 cells/well of a 96-well culture plate, 3 × 105 cells/well of a six-well culture plate), they were allowed to attach for 24 hours, and the media was removed. Cells were washed with D-PBS, and then DMEM:F-12 serum depletion media was replaced with 0.001%, 0.005%, 0.01%, or 0.02% BAC, bimatoprost 0.01% (Lumigan; Allergan, Irvine, CA, USA), latanoprost 0.005% (Xalatan; Pfizer, New York, NY, USA), travoprost 0.004% (Travatan, Alcon), or taf luprost 0.0015% with/without 0.001% BAC (Taflotan/Taflotan-s; Santen, Osaka, Japan). The cells were exposed to the drugs for 15 or 30 minutes [2,16,17]. The stimuli were removed after incubation. The cells were washed again with D-PBS and then transferred to a serum-free DMEM:F-12 media. Cells were incubated for an additional six hours to stabilize. The control solution was the same volume of PBS added into the cells.
Cell morphology analysis
Treated cells were observed after 30 minutes of drug treatment and upon removing the stimuli. Cells were washed again with PBS and then transferred to serum-free DMEM:F-12 media. Cells were incubated for an additional six hours to stabilize. Finally, the morphological features of the cultured cells in six-well plates were observed by phase-contrast microscopy [18].
Cell viability assay
The Cell Counting Kit-8 (CCK-8) assay was used as a qualitative index of cell viability. The CCK-8 assay was used to measure cytotoxicity under starved conditions, which were based on the conversion of a water-soluble tetrazolium salt, 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt (WST-8), to a water-soluble formazan dye upon reduction by dehydrogenases in the presence of an electron carrier [19,20]. Cells were plated in 96-well microtiter plates at a density of 6 × 103 cells per well, then the live cell count was obtained using CCK-8 (Dojindo, Sunnyvale, CA, USA) according to the manufacturer's protocol. In brief, 10 µL of CCK-8 solution was added to each well, and the samples were incubated for two hours before the absorbance was measured at 450 nm.
Fluorescent activated cell sorting analysis
Cells (1 × 106) were suspended in 100 µL of PBS, followed by 200 µL of 95% ethanol while vortexing. Then, the cells were incubated at 4℃ for 1 hour, washed with PBS, and resuspended in 250 µL of 1.12% sodium citrate buffer (pH 8.4) together with 12.5 µg of RNase. Incubation proceeded at 37℃ for 30 minutes. The nuclei were then stained by applying 250 µL of propidium iodide (50 µg/mL) for 30 minutes at room temperature. Nuclei displaying hypodiploid sub-G1 DNA contents were identified as apoptotic. The stained cells were analyzed by fluorescent activated cell sorting (FACS) on a FACScan flow cytometer (FACScalibur; BectonDickinson Biosciences, San Jose, CA, USA) to determine relative DNA content based on fluorescence. The sample of each group included with more than 10,000 individual cells [21].
Statistical analysis
Statistical comparisons were performed with SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA). The cell viability and apoptosis rates were analyzed by Mann-Whitney U-test.
Results
Morphological change of cells
To investigate the cytotoxicity of BAC at different concentrations in cultured human conjunctival fibroblast cells, we observed morphological changes by phase-contrast microscopy. Cells were treated with BAC at different concentrations (0.001%, 0.005%, 0.01%, and 0.02%) for 30 minutes. BAC caused apoptotic characteristics, such as cell shrinkage and detachment from the plate, in a dose-dependent manner (Fig. 1).
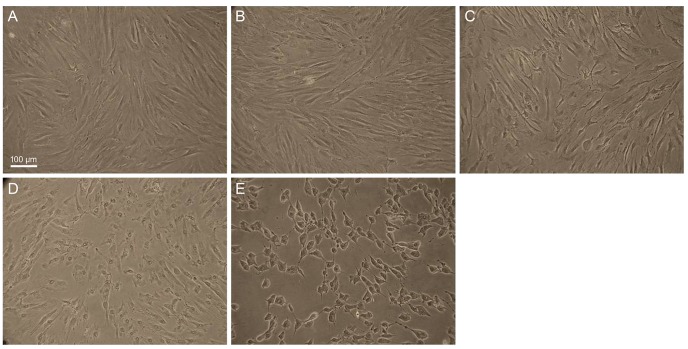
The morphological features of cultured human conjunctival fibroblast cells with different concentrations of benzalkonium chloride (BAC) in six-well plates were observed by phase-contrast microscopy (×100). (A) Control, (B) 0.001% BAC, (C) 0.005% BAC, (D) 0.01% BAC, and (E) 0.02% BAC. Cell shirnkage increased dose dependently with BAC concentration. Scale bar, 200 µm.
We investigated whether the treatment with different prostaglandin formulations affected the induction of apoptosis in cultured human conjunctival fibroblast cells. Morphological changes, including apoptosis, were observed in cultured cells treated with bimatoprost 0.01% (with BAC 0.02%) and latanoprost 0.005% (with BAC 0.02%). Also, in the tafluprost 0.0015% group (with BAC 0.001%), mild cell shrinkage was visible. However, cultured conjunctival fibroblast cells showed a non-toxic effect to travoprost 0.004% (with 0.001% Polyquad) and preservative-free tafluprost 0.0015% treatment, e.g., cell shrinkage and detachment from the plate were comparable with those of the control group (Fig. 2).

The morphological features of cultured human conjunctival fibroblast cells with different prostaglandin formulations in six-well plates were observed by phase-contrast microscopy. (A) Control, (B) bimatoprost 0.01% (benzalkonium chloride [BAC] 0.02%), (C) latanoprost 0.005% (BAC 0.02%), (D) travoprost 0.004% (0.001% Polyquad), (E) tafloprost 0.0015% (BAC 0.001%), and (F) tafluprost 0.0015% (preservative-free). Compared with the control group, severe cell shrinkage was observed in bimatoprost 0.01% (BAC 0.02%) and latanoprost 0.005% (BAC 0.02%) groups. In the tafluprost 0.0015% group (BAC 0.001%), mild cell shrinkage was visible. In contrast, the travoprost 0.004% (0.001% Polyquad) and preservative-free tafluprost 0.0015% groups showed relatively similar cell morphology to that observed with the control group. Scale bar, 200 µm.
Cell viability
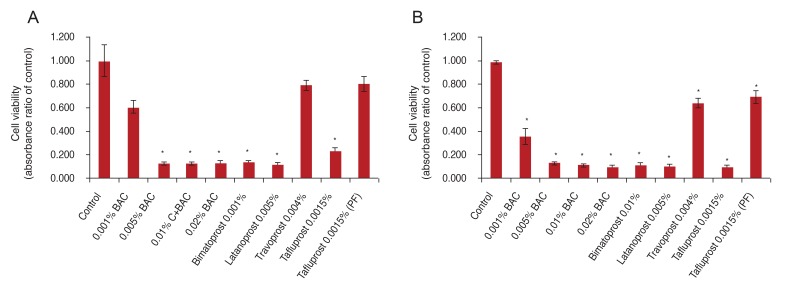
To investigate whether BAC or prostaglandin formulations reduce cell viability, the cells were exposed to the drugs for 15 or 30 minutes and then analyzed by CCK-8 assay. The sample absorbance was measured at 450 nm. CCK-8 assay showed that cell viability of cultured conjunctival fibroblast cells was decreased by treatment with BAC alone or BAC-containing anti-glaucoma drugs. In 15 minutes, the absorbance rates (of control) were 62.8% to 12.8% upon BAC treatment at different concentrations, 13.1% and 11.5% in PGs with BAC (bimatoprost, latanoprost), 22.6% in tafluprost with a small amount of BAC, 78.8% in preservative-free tafluprost, and 78.8% in alternatively preserved PG (travoprost with Polyquad). BAC at different concentrations (0.005% to 0.02%), PGs with BAC (bimatoprost, latanoprost) and taf luprost with a small amount of BAC significantly decreased cell viability compared with the control group (p < 0.05). In contrast, 0.001% BAC, alternatively preserved PG (travoprost with Polyquad), and preservative-free tafluprost groups had slightly decreased cell viability compared to the control group (Fig. 3A). In 30 minutes, the absorbance rates (of control) were 36.2% to 10.3% upon BAC treatment at different concentrations, 11.8% and 11.1% in PGs with higher BAC concentrations (bimatoprost, latanoprost), 10.2% with tafluprost and a small amount of BAC, 70.1% with preservative-free taf luprost, and 64.8% with alternatively preserved PG (travoprost with Polyquad). All groups showed a significantly decreased cell viability compared with the control group (p < 0.05). However, the preservative-free tafluprost and travoprost with Polyquad groups showed a lower cytotoxicity than the other drugs (Fig. 3B).
Apoptosis analysis
Flow cytometric and quantitative analyses of apoptotic cells were performed after culture for 30 minutes. Apoptosis was analyzed as a sub-G1 fraction by FACS analysis. The apoptosis rates were 13.4% in control, 22.7% in 0.005% BAC, 43.7% in 0.02% BAC, 42.5% and 37.0% in PGs with more BAC (bimatoprost, latanoprost), 21.0% in tafluprost with a small amount of BAC, 13.8% in preservative-free tafluprost, and 10.8% in alternatively preserved PG (travoprost with Polyquad) (Fig. 4A). The groups treated with 0.005% BAC, 0.02% BAC, PGs with more BAC (bimatoprost, latanoprost) and tafluprost with small amount of BAC showed significantly higher apoptosis rates than the control group (p < 0.05). However, preservative-free tafluprost and alternatively preserved PG (travoprost with Polyquad) groups showed similar rates to the control group (Fig. 4B).
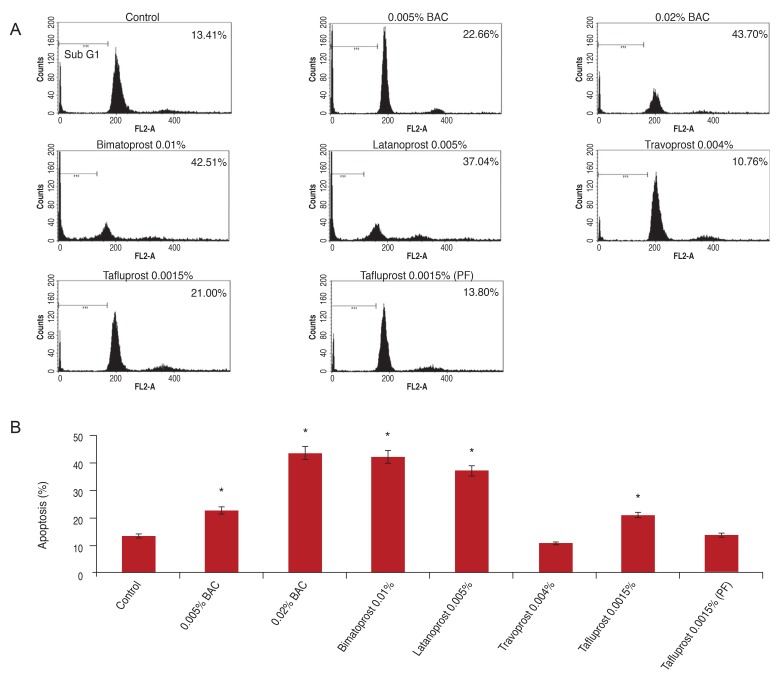
Flow cytometric and quantitative analyses of apoptotic cells after culture for 30 minutes. Apoptosis was analyzed as a sub-G1 fraction by fluorescent activated cell sorting analysis (A) and columns (B). *p < 0.05 vs. corresponding value for control by Mann-Whitney U-test. BAC = benzalkonium chloride; PF = preservative-free.
Discussion
BAC is the most commonly used preservative for antimicrobial action. Several studies revealed ocular surface toxicity resulting from BAC present in ophthalmic agents [1-11,17,22-28]. Also, in this study, we observed increased cellular shrinkage and apoptosis, and reduced cell viability in BAC-containing agents compared to BAC-free agents. Upon increasing the concentrations of BAC (0.001%, 0.005%, 0.01% and 0.02%), the cytotoxicity was also increased, as noted in other studies (Fig. 1) [3,5,11].
We next determined whether these toxicity were due to the toxicity of BAC or the toxicity of the drug itself. We investigated a the newly released drug, bimatoprost 0.01% (with BAC 0.02%), at a low concentration but with a relatively high concentration of BAC (with BAC 0.05%) to reduce the conjunctival hyperemic side effect. The cell viability of bimatoprost 0.01% (with BAC 0.02%) was 13.1% of the control value in 15 minutes and 11.8% of the control value in 30 minutes, while latanoprost 0.005% (with BAC 0.02%) was 11.5% of the control in 15 minutes and 11.1% of the control in 30 minutes. However, that of BAC 0.02% only was 12.8% of the control in 15 minutes and 10.3% of the control in 30 minutes. So, we can presume there is minimal effect of bimatoprost and latanoprost on conjunctival fibroblast cells. In the case of tafluprost 0.0015% (with BAC 0.001%), the cell viability was 22.6% of the control in 15 minutes and 10.2% of the control in 30 minutes, but that of BAC 0.001% showed 62.8% viability of the control in 15 minutes and 36.2% of the control in 30 minutes. As a result, we can presume the relative toxicity of tafluprost.
We also studied preservative-free PGs using tafluprost 0.0015%. The minimum concentration of BAC 0.001% was more cytotoxic than tafluprost 0.0015% (preservative-free) as shown in Fig. 3. Even the small amount of BAC showed cytotoxicity; therefore, considering the long term effects on the ocular surface when using anti-glaucomatic agents and artificial tears, preservative-free agents are of great interest.
There is increased interest for alternative preservatives to reduce the cytotoxicity of BAC. Several studies have reported that alternative preservatives, such as Sofzia, Purite and Polyquad are less toxic than BAC [1-3,14,17,26,27]. Also, in this study, Fig. 3 shows the viability of cells treated with travoprost 0.004% containing Polyquad was 78.8% of that of the control in 15 minutes and 64.8% of that of the control in 30 minutes, while that of tafluprost 0.0015% (preservative-free) was 78.8% of the control in 15 minutes and 70.1% of the control in 30 minutes. The quaternary ammonium compound Polyquad has been used by Alcon as a preservative in artificial tears since 1987 and acts as a surfactant, disrupting bacterial cell membranes and ultimately leading to bacterial cell death. Polyquad is a cationic polymer of many quaternary ammonium structures with a 27-fold higher molecular weight than BAK. It lacks a hydrophobic region and, as a result, has no surfactant/detergent properties. Due to these two chemical properties, Polyquad is unable to penetrate mammalian cells and cause cytotoxic effects [22]. Ammar et al. [3] reported the cytotoxicity of travoprost 0.004% with BAC, travoprost 0.004% with Polyquad, and travoprost 0.004% with SofZia. The observed relative cytotoxicity values were Polyquad < SofZia < BAC, and each drug offered more protection to live conjunctival cells than preservatives only, e.g., Travatan 0.004% has a protective effect on cells. We also studied only travoprost 0.004% with Polyquad in the travoprost 0.004% group, where showed a lower cytotoxicity than other drugs. However, it has the limitation that it was not compared in combination with other preservatives.
The benefits of preservative-free eye drops are already known from several studies, but they also have drawbacks. The biggest drawback is the inconvenience of use for older people who often suffer from glaucoma and dry eye. Also, preservative-free eye drops are more expensive than preservative-containing eye drops. Long-term use of these agents is also a burden. In addition, the action of preservatives for improving corneal penetration is also limited in preservative-free eye drops. Further study is needed regarding alternative preservatives that have a lower cytotoxicity than BAC.
Obviously, these results obtained via an in vitro model cannot be fully extrapolated to in vivo conditions, due to anti-inflammatory factors, and the diluting action of tears and eyelid blinking. However, our experimental results were in good agreement with data from other studies assessing the toxic side effects of preservative induced at the ocular surface [1-11]. Moreover, we observed effects at 15 and 30 minute exposure times, so the results of long-term exposure to these agents cannot be extrapolated.
In conclusion, this in vitro study revealed that BAC-induced cytotoxicity is dose-dependent, although it is important to emphasize that the clinical significance of toxicity differences observed among the differnet PG formulations has not yet been firmly established. Alternatively preserved or preservative-free glaucoma medications seem to be a reasonable and viable alternative to those preserved with BAC. More studies are needed to better understand how in vitro findings compare to in vivo responses.
Notes
No potential conflict of interest relevant to this article was reported.