The Therapeutic Effects of Angiotensin-Converting Enzyme Inhibitors in Severe Non-proliferative Diabetic Retinopathy
Article information
Abstract
Purpose
To evaluate the effects of angiotensin-converting enzyme inhibitors (ACE-I) in retarding progression of severe non-proliferative diabetic retinopathy (NPDR) in normotensive type 2 diabetic patients.
Methods
This was a retrospective case control study of 128 patients with normotensive type 2 diabetes with lower than +1 dipstick proteinuria and severe NPDR who were classified into either an ACE-I treated group (Enalapril maleate 10 mg, n=12 , Ramipril 5 mg, n=17) or an ACE-I untreated group (n=99). Medical records were reviewed for endpoints of (a) occurrence of proliferative diabetic retinopathy (PDR) or macular edema (ME) for which laser phototherapy was necessary or (b) development of proteinuria of higher than +1 level requiring medication of ACE-I.
Results
From the total of 128 patients, there were 29 ACE-I treated patients and 99 ACE-I untreated patients. There were no differences in the average age, duration of diabetes, body mass indices, blood pressure and levels of hyperglycemia or HbA1C between the two groups. Blood pressure and HbA1C levels in both groups remained unchanged during the study. The mean follow-up period was 41.6 months. In the ACE-I group, 6 patients progressed to PDR, 5 to ME and 6 developed proteinuria of greater than +1 over the follow-up period. In the control group, 30 patients progressed to PDR, 6 to ME and 9 developed proteinuria of greater than +1 over the follow-up period.
Conclusions
Small doses of ACE-I did not yield any beneficial effects in retarding the progression of severe NPDR.
Diabetic retinopathy is one of the main causes of vision loss, even in developed countries.1 Even though the etiology of diabetic retinopathy has yet to completely defined, it is widely accepted that the control of risk factors, such as blood sugar and blood pressure level, retards the progress of this disease.2,3 Although laser photocoagulation is recognized as a definitive method for slowing down the aggravation of severe non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR), it is accompanied by complications including pain, reduced vision, narrowed visual field, macular edema, macular coagulation, choroidal detachment, exudative retinal detachment and vitreous hemorrhage, and in rare cases, damage to other structures in the eye.4 Therefore, clinical studies are underway to find non-invasive methods for the treatment of diabetic retinopathy. Recently, the rennin-angiotensin system (RAS) was found to interfere with the development of PDR,5 consistent with all the RAS components being found in the retina, and the densities of prorenin, renin, angiotensine II increasing in the vitreous of patients with PDR and macular edema (ME).5-10 Further, patients with diabetic retinopathy had higher levels of angiotensin-converting enzyme (ACE),11 and the angiotensine II formed by ACE promotes the expression of growth factors, such as vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF) and platelet-derived growth factor (PDGF), which in turn result in formation of new blood vessels and increased permeability of blood vessels and oxidative stress.5,7,12,13 As a result, angiotensin-converting enzyme inhibitor (ACE-I) can prevent destruction of blood retina barrier (BRB)5,12 and retard progression of the disease to PDR.14
A few reports addressed the effects of ACE-I on the patients with diabetic retinopathy but the authors differed in their interpretation of the data. Consequently, our clinical research sought to clarify preventive effects of ACE-I on the aggravation process from NPDR to PDR in patients with type 2 diabetes.
Materials and Methods
Of 2,528 patients who had been diagnosed with type 2 diabetes at our hospital from January 1998 to December 2002, we performed retrospective reviews on 240 of them who had been under continuous observation for over 10 years by the Departments of Ophthalmology and Endocrinology & Metabolism with visits every six months until December 2004. A diagnosis of type 2 diabetes was determined by personal medical history, positive test results, physical examination, and clinical manifestations in at least two follow-up visits. Among these type-2 diabetics, we excluded patients who maintained normal blood pressure of under 140/90 mmHg without anti-hypertensive drugs and selected the ones diagnosed with severe NPDR confirmed by two successive examinations of the fundus at least 6 months apart. We selected 128 patients with normal blood creatinine levels, who tested negative or less than +1 in dipstick test for proteinuria, and with a maximum corrected vision of greater than 20/50. We also eliminated patients with a past history of general diseases other than diabetes or ones who received eye surgery other than for cataracts. After sorting our final test subjects into one of two groups, one group medicated with ACE-I (the Test Group) and the other without ACE-I treatment (the Control Group), we compared the fundus examination results and clinical data between the two groups. The ACE-I drugs used were of 2 types: Enalapril maleate 10 mg (Renatone®), and Ramipril 5 mg (Tritrace®).
Examination of the fundus was performed with a 78D (Volk lens, superfield) lens, slit lamp microscope and indirect ophthalmoscope following pupillary dilatation with phenylephrine HCL 2.5% (Mydfrin) and tropicamide 1% (Mydriacyl) administered 3 times, 15 minutes apart. Through examination of the fundus, diabetic retinopathy was classified as mild, moderate or severe phases, in accordance with the 40-50 levels in the modified Arlie House classification,15 and confirmed by fluorescein fundus angiography (FAG). We checked blood creatinine, HbA1C and proteinuria levels and their maximum corrected vision. During the follow up period, photographs of the fundus were taken once every year regardless of the slit lamp microscopy results. If severe diabetic retinopathy with a 60-80 level by modified Airlie House classification or macula edema was observed through slit lamp microscopy, the FAG was performed again to confirm the diagnosis.15 The terminating criteria for patients in this study were defined as aggravation of severe NPDR into PDR requiring laser photo-coagulation treatment, occurrence of macula edema, observed through fundus examination and FAG, or positive results of a proteinuria test with levels higher than +1 requiring medication by ACE-I.
SPSS 11.0 was used for statistical analysis, while Student's t-test and Chi-square methods were used for comparison of earlier data and termination points between the Test Group and the Comparison Group. The paired t-test was used for the comparison of the changes in the primary and the final mean arterial pressure in the two groups. The results of these statistical tests were considered meaningful only when the p-value was less than 0.05.
Results
The average age of the 128 patients was 61.7±3.9 (range, 51 to 78 years), and the patients consisted of 63 men and 65 women. The group medicated with ACE-I (Group 1) was composed of 29 patients, and the Control Group (Group 2) had 99 patients. For the two ACE-I drugs, 10 mg of Enalapril maleate 10 mg (Renatone®) was administered to 12 patients, while 5 mg of Ramipril 5 mg (Tritrace®) was given to 17 patients.
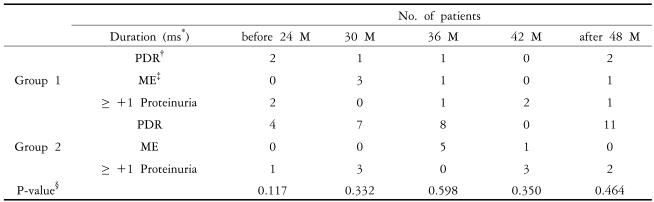
The average age of Group 1 was 59.1±2.5, with 11 men (37.9%) who had diabetes for an average of 15.5±2.7 years. Among the patients of Group 1, 9 were treated with oral medications (pills), 10 were treated with insulin, and 10 were treated with oral medication and insulin. The blood creatinine levels were 0.78±0.08 mmHg, and HbA1c levels were 10.4 ±0.9%. The average age of Group 2 was 62.5±4.0, with 52 men (52.5%) who had diabetes for an average of 16.4± 3.3 years. Among the patients of Group 2 , 38 were treated with oral medications (pills), 38 were treated with insulin, and 23 were treated with oral medications and insulin. The blood creatinine levels were 0.83±0.11 mmHg, and HbA1c levels were 10.0±1.2%. There were no significant differences between two groups in the male/female ratio, how long the patients had diabetes, or in blood pressure readings. Otherwise, the age (p=0.00) and serum creatinine levels (0.039) in Group 2 were higher than those in Group 1, and the body mass index (BMI) (p=0.005) and HbA1c (p=0.045) values of Group 1 were higher than those in Group 2 (Table 1). In Group 1, 6 patients showed aggravation of severe NPDR into PDR, requiring laser photo-coagulation treatment, macula edema occurred in 5 patients, and 6 patients showed positive results of proteinuria, with levels greater than +1. In Group 2, 30 patients showed aggravation of severe NPDR into PDR requiring laser photo-coagulation treatment, macula edema occurred in 6 patients, and 9 patients showed positive results of proteinuria, with levels greater than +1. Taken together, 62 patients out of the original pool of 128 satisfied the terminating criteria of this study during the observation period of the average 41.6±16.3 months. The average observation period was 37.4±14.0 months in Group 1 and 42.9±16.8 months in Group 2, with no significant difference between the two groups (P=0.115). In Group 1, severe NPDR was aggravated into PDR in 6 patients (20.7%), macular edema occurred in 5 patients (17.2%), and proteinuria values rose to over 1+ in 6 patients (20.7%). In Group 2, severe NPDR was aggravated into PDR in 30 patients (30.3%), ME occurred in 6 patients (6.1%), and proteinuria values rose to over 1+ in 9 patients (9.1%) (Table 2, Fig. 1).

Patients reaching study endpoints.
*Group 1 : ACE-I treated group.
†PDR : proliferative diabetic retinopathy.
‡ME : macular edema.
§PRO : ≥ 1+ Proteinuria.
ΠGroup 2 : Control group (ACE-I untreated).
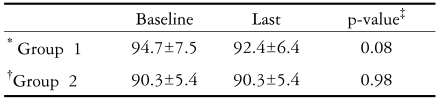
In Group 1, the average arterial pressure was 94.7±7.5 mmHg at the first examination, and 92.4±6.4 mmHg at the final examination (P=0.075). In Group 2, the average arterial pressure was 90.3±5.4 mmHg at the first examination, and 90.3±5.4 mmHg at the final examination (P=0.98), with no significant differences between the two groups.
Discussion
It is well established that early diagnosis and treatment of diabetes can reduce the chance of blindness caused by diabetes and that rigorous control of blood sugar and hypertension retards occurrence and progress of diabetic retinopathy.16-18 Recently, it was found that controlling RAS affects the onset and progress of diabetic retinopathy.5-10 Chaturvedi et al14 performed a compare and contrast study of EUCLID (EURODIAB Controlled Trial of Lisinopril in Insulin-Dependent Diabetes Mellitus) on type 1 diabetes and showed that administration of Lisinopril reduced progress of diabetic retinopathy by 50%, with an 80% reduction of aggravation into PDR in comparison with the placebo group (P<0.02) after two years.8 These studies showed that HbA1c was 0.4% lower in the test group where the Lisinopril had been administered, but there were no significant differences between the two groups (P<0.06). These results suggest that well-controlled blood sugar in the group taking ACE-I before the study may have accounted for the improved results in diabetic retinopathy. As for effects on blood pressure, only a small decrease of 3 mmHg was observed after one month from the administration of Lisinopril14 Jackson et al.19 published a clinical study on the therapeutic effect of ACE-I on diabetic retinopathy without the control group. Out of 10 patients with 20 eyes with type 1 diabetes, 9 eyes exhibited aggravation; 4 eyes did not show any changes, and 7 eyes showed improvements in diabetic retinopathy over 2 years. These outcomes on the therapeutic effect of ACE-I are not sufficient to affirm whether the slowdown of progression of diabetic retinopathy was due to the decrease of blood pressure or to RAS. Therefore, the therapeutic effect of ACE-I on diabetic retinopathy patients with normal blood pressure was subsequently studied. Rachmani et al.20 observed 250 non-hypertensive type 2 diabetic patients who had not reported any problems with diabetic retinopathy. Among these 250 patients, a test group of 126 patients were given 10 mg of Enalapril on a daily basis, and the control group of 124 patients were given a placebo. These two groups of patients were tracked for 5 to 6 years, and diabetic retinopathy occurred to 9 patients (7.1%) in the test group, while 23 (18.5%) patients in the control group showed an 11.5% reduction in absolute risk level.
While several reports have covered the retarding effect of ACE-I on the occurrence of diabetic retinopathy and aggravation of mild NPDR, there have been few reports on the therapeutic effect of ACE-I on severe NPDR related to aggravation into PDR. We showed that the there was no statistically meaningful effects of ACE-I in non-hypertensive (normotensive) type 2 diabetic patients for retarding the progression of severe NPDR into PDR over our average observation period of 41.6+16.3 months (Table 2). Eight out of 29 patients administered reached one of the pre-determined termination points of this clinical study within 36 months of administration. This result is similar to that reported by Pradhan et al.21 who studied 35 type 2 diabetic patients with normal blood pressure. These 35 patients were classified into one of 2 groups at random, the test group of 18 patients who were given 5 mg of Enalapril and the control group of 17 patients who were given a placebo (multivitamin) for an average observation period of 7.2 months. This study was discontinued, as no meaningful differences were found between the two groups.
In addition, we showed that PDR occurred to 6 medicated patients from Group 1 (20.7%) and 30 non-medicated patients from Group 2 (30.3%), supporting the partial retarding effect of ACE-I on the aggravation of the diabetic retinopathy to PDR. However, overall result of the experiment in terms of reaching the pre-determined termination point did not show significant differences between the two groups (Fig. 1). Among the 11 patients who acquired macular edema at the end of this study, none were compromised temporarily or permanently following cataract surgery.
Though this was a retrospective clinical study, we encountered the following limitations: It was difficult to match the patients in the two different groups by gender, age and other factors. The BMI and HbA1c in Group 1 were higher than those in Group 2 and the age and serum creatinine levels in Group 2 were higher than those in Group 1. Although serum creatinine levels between the two groups were different, it was meaningless, because serum creatinine levels of all the subjects in this study were within normal range. The mean BMI values between two groups were different, but both values were in the overweight range, and so the mean BMI values were also such that they were also not meaningful in this study. Short-term fluctuations in blood sugar level were inevitable due to the long duration of diabetes. In addition, other variable factors (for example,. physical exercise, dietetic therapy, and effects on diabetic retinopathy affected by the improvement of renal and cardiac function after administration of ACE-I) were difficult to control. During the retrospective observation period, average HbA1c levels, which reflects control of blood sugar, remained as high as greater than 10%, suggesting that blood sugar had been out of control for a long period of time. This seems to be supporting the inevitability of fluctuation of short-term blood sugar levels and the difficulties in keeping the blood sugar level under control for the patients who have passed more than 10 years after being diagnosed with diabetes. Taking this fact into consideration, Orchard et al22 determined the relationship between cumulative glycemic exposure, which reflects both the level and duration of hyperglycemia, and micro-vascular complications. In this study, "A1 months", which is the result of multiplying HbA1c level higher than normal by time lag in months between the measurements, was used to reflect the level of excess glycosylated Hemoglobin in a certain time period. This method seems to be meaningful in the short period of most clinical studies.
It is possible that the small amount of ACE-I used in this clinical study may have failed to keep the RAS under control. However, the actual prescribed amount used in general practice has been recorded, and follow-up studies are warranted.
Even though we did not find the retarding or preventative effects of ACE-I on diabetic retinopathy in this study, long-term, massive and prospective clinical studies with stricter variants (for example, age, gender, serum creatinine level, HbA1c, BMI, and MAP) to prevent the statistical biases are still warranted in the future, as diabetes is a long-term disease.