Peripheral Anterior Synechiae and Ultrasound Biomicroscopic Parameters in Angle-Closure Glaucoma Suspects
Article information
Abstract
Purpose
To investigate the correlation between peripheral anterior synechia (PAS) and the quantitative anterior chamber angle parameters measured by ultrasound microscopy (UBM) in angle-closure glaucoma suspect (ACGS) eyes.
Methods
Eyes were defined ACGS as having occludable angles and intraocular pressure less than 21 mm Hg without glaucomatous optic nerve head. The gonioscopic criteria for ACGS were the trabecular meshwork invisible in 3 or more quadrants of the entire angle and the angular width less than 20 degrees by Shaffer classification. Twenty-seven eyes of 20 patients underwent anterior chamber angle and ciliary body imaging with UBM. Angle opening distance (AOD500), angle recess area (ARA), trabecular-ciliary process distance (TCPD) and trabecular-iris angle (TIA) were measured from the UBM images at each quadrant.
Results
The AOD500, ARA, and TIA were not significantly different between the eyes with PAS (9 eyes)and without PAS (18 eyes) at each quadrant. However, the TCPD was significantly shorter in the superior quadrant when compared with the eyes without PAS (mean: 405.3±70.9 µm vs 536.4±140.5 µm) (p<0.05).
Conclusions
The results suggest that the shorter distance from trabecular meshwork to ciliary body or the anterior placement of ciliary process may play a role in the development of PAS in ACGS eyes.
Peripheral anterior synechia (PAS) is a condition in which the iris adheres to the angle, and it is one of the pathognomonic signs of primary angle-closure glaucoma (PACG). PAS may be seen in other ocular conditions such as uveitis and iris neovascularization, and it can also develop within a week after shallow to flat anterior chamber following intraocular surgery.1,2
How PAS develops in an eye is not fully understood. Prolonged apposition and repeated angle closure attacks may lead to the development of PAS. The extent of PAS may be related to the visual field damage, larger vertical cup-to-disc ratio, and higher untreated intraocular pressure (IOP).3,4 The characteristics of iris, such as contour, thickness and adhesiveness may also be associated with the formation of PAS. However, the exact mechanism of PAS formation still remains to be determined.
There have been several reports that extension of PAS occurred in 13~30% of PACG eyes after laser iridotomy.5,6 Choi and Kim also reported on PAS progression after iridotomy, and found that presence of plateau iris and high IOP before iridotomy were positively correlated with PAS progression.7 Aside from the pressure component, the structural characteristics of anterior chamber angles may predispose to the development of PAS. In this study, we evaluated the structural characteristics of anterior chamber angles measured with UBM in angle-closure glaucoma suspects (ACGS) eyes and investigated their association with the development of PAS.
Materials and Methods
This was a retrospective cross-sectional study. According to the Kim and Jung's classification system for PACG, we defined ACGS as an eye having IOP<21 mmHg, no glaucomatous optic nerve head signs and an occludable angle.8 The gonioscopic criteria for an occludable angle were: 1) the trabecular meshwork was invisible in 270° or more of the entire angle in the primary position of gaze without indentation and/ or 2) the angular width was less than 20 degrees by the Shaffer grading. Exclusion criteria were: 1) presence of other PAS-associated disorders like iris neovascularization and uveitis; and 2) a history of prior intraocular surgery or use of anti-glaucomatous medication. Only patients with ACGS were enrolled in order to minimize the possible IOP-related effects on PAS formation. Fellow eyes of the patients with unilateral PACG were also included if they satisfied the above criteria. Twenty-seven eyes from 20 Korean patients who were examined at the glaucoma clinic at Korea University Guro Hospital between April 2005 and September 2005 and who met the aforementioned criteria were retrospectively reviewed. Approval of the institutional review board was not needed when this study was carried out because all of the measurements were obtained by the methods routinely used in patients with a shallow anterior chamber and this study did not include surgical or medical interventions.
All patients underwent routine slit-lamp examination including IOP measurement and gonioscopy. One investigator (YYK) performed dynamic gonioscopy using a Zeiss 4-mirror contact lens in a darkened room with minimum-possible slit-lamp illumination, taking care that the slit beam did not impinge upon the pupil. PAS was considered to be present when the adhesion of the iris reached the mid-trabecular meshwork and its extent exceeded one clock hour on indentation gonioscopy.
UBM images were acquired from the eyes before laser iridotomy if it was performed. Ultrasound biomicroscopy was performed with the commercial model of the instrument (P45 Ultrasound BioMicroscope®, Paradigm Medical Industries, Salt Lake City, UT, USA) and a 50 MHz transducer.
Under topical anesthesia, a plastic saline-containing eye-cup was used to separate the eyelids. Radial scans of 12, 3, 6 and 9 o'clock positions and an axial scan were performed under standard room illumination. UBM examination was performed with the patient lying supine. Five parameters were measured with UBM according to the Pavlin et al's method.9-11 Figure 1 shows a schematic representation of the UBM measurements. Anterior chamber depth (ACD) indicates the distance between the endothelium and the anterior surface of the lens along the visual axis. Angle opening distance (AOD500) corresponds to the distance between the trabecular meshwork and the iris at 500 µm anterior to the scleral spur. Trabecular iris angle (TIA) is defined as an angle formed with the apex at the iris recess and the arms passing through the point on the meshwork 500 µm from the scleral spur and the point on the iris perpendicularly opposite. Trabecular ciliary process distance (TCPD) indicates the distance between the trabecular meshwork and the ciliary process at 500 µm anterior to the scleral spur. Angle recess area (ARA) represents the triangular area bordered by the anterior iris surface, corneal endothelium, and a line perpendicular to the corneal endothelium drawn to the iris surface from a point 750 µm anterior to the scleral spur. All these quantitative measurements were performed by a single well-trained operator (JHO).
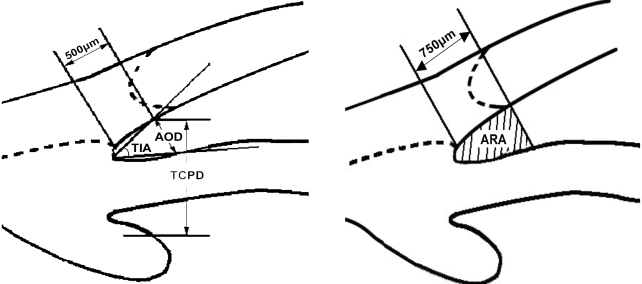
Schematic representation of UBM anterior chamber angle measurement. Angle opening distance (AOD500) is defined as the length of the line drawn from the point on the corneal endothelial surface 500 µm anterior to the scleral spur to the iris surface perpendicular to the corneal endothelial surface. Trabecular-iris angle (TIA) is defined as an angle formed with the apex at the iris recess and the arms passing through the point on the meshwork 500 µm from the scleral spur and the point on the iris perpendicularly opposite. Trabecular ciliary process distance (TCPD) is defined as the distance between a point 500 µm from the scleral spur and the ciliary process on the line that is perpendicular through the iris. Angle recess area (ARA) is defined as the triangular area bordered by the anterior iris surface, corneal endothelium, and a line perpendicular to the corneal endothelium drawn to the iris surface from a point 750 µm anterior to the scleral spur.
Statistical analysis was performed using a Mann-Whitney test. A P-value less than 0.05 was considered to be significant.
Results
Twenty-seven eyes of 20 patients (2 males and 18 females) were evaluated. Of these 27 eyes, gonioscopy revealed PAS in 9 (Table 1). PAS was localized to one quadrant in 7 eyes or extended to two quadrants in 2 eyes. Four eyes had PAS in the superior quadrant; 3, inferior; 2, nasal; and 2, temporal. The mean age was 66.1±11.4 years among the patients having eyes with PAS and 63.1±10.0 years among those without PAS (P=0.40). The mean IOP was 13.0±2.7 mmHg among the PAS-positive eyes and 11.9±3.4 mmHg among the PAS-negative ones (P=0.43). Among the 27 eyes studied, 10 were fellow eyes of patients with unilateral acute PACG. Age, gender ratio, clinical types, and IOP were similar between these two groups (Table 1).
The anterior chamber angle parameters measured with UBM are presented in Table 2. Anterior chamber depth was not significantly different between the two groups (mean: 2.11±0.27 mm, PAS-positive; 2.07±0.31 mm, PAS-negative; P=0.74).
The AOD500, TIA and ARA showed no statistically significant difference between the PAS-positive group and the PAS-negative group. However, the TCPD was significantly shorter in the superior quadrant in the PAS-positive group than in the PAS-negative group (478.4±74.9 µm vs 547.3±138.0 µm P<0.05)
Discussion
Gonioscopy is an indispensable part of examination in assessing glaucoma patients since it can inform clinicians of the type of glaucoma and also the guidelines for treatment. The presence of PAS is one of the valuable gonioscopic signs by which we can make a diagnosis of angle closure.
The development of ultrasound imaging technology has revealed much about anterior chamber angle structures as well as their clinical implications. However, the exact mechanism of PAS formation is still uncertain and the literature has few UBM studies on the relationship between angle parameters and the development of PAS.
It is acknowledged that formation of PAS starts as the peripheral part of iris first adheres to Schwalbe's line or to the angle recess. Gorin proposed two theories as to the mode of development of PAS.12 According to the first theory, the peripheral iris sticks to Schwalbe's line and then the PAS extends out toward the angle recess. According to the second theory which he named "shortening of the angle", the peripheral iris first attaches to the angle recess and then the PAS extends out toward the Schwalbe's line. Gorin believed that the first theory best describes the development of angle closure in most cases. Lowe preferred the term "creeping angle-closure" for the second theory and mentioned that this is more descriptive of the insidious clinical course of most primary angle closure glaucoma; he considered it to be the most common mode of angle closure in Asians.13,14 Later, Sakuma et al reported that two thirds of appositional closures started from the Schwalbe's line as in the first theory.15 Inoue et al. proposed that the second theory is more probable because 70% of the PAS present in their acute PACG cases were incomplete ones which were adherent up to the mid meshwork.1
Concerning the location of PAS, Phillips,16,17 Bhargava18 and Inoue1 all independently reported that it is found most frequently in the superior sector, and that this is due to the relatively narrow angle of this sector in normal eyes. Gonioscopically, anterior chamber angle is known to be narrower in the superior quadrant than it is elsewhere.16,17,19,20 The superior and inferior angles were reported to be narrower than the nasal and temporal angles when measured by UBM.21 It is believed that the superior portion of the angle is the earliest site of synechial occlusion.22
Our study demonstrates that the TCPD in the superior quadrant differed significantly in ACGS eyes with PAS compared with those without PAS. This difference seems to be associated with relatively anteriorly positioned ciliary processes. With the aid of UBM, many researchers have recently found that a plateau iris is correlated with an anteriorly placed ciliary process. A plateau iris may be related to PAS progression despite a patent iridotomy in angle-closure glaucoma patients.7 Theoretically, the anteriorly placed ciliary process may cause crowding of the angle structures, resulting in PAS formation. For example, in an eye with an anteriorly placed ciliary process, a thickened iris under dark illumination could broaden the contact between the iris and the angle. This broadened contact could possibly encourage PAS formation and, as this continues, lead to creeping angle closure, a condition in which the PAS slowly advances forward circumferentially, moving the iris insertion gradually more forward onto the trabecular meshwork. Yeung et al. studied the prevalence and mechanism of appositional angle closure in acute primary angle closure after iridotomy.23 They reported that appositional angle closure occurred after laser iridotomy in 55.6% of their cases and that an important difference was found in the TCPD between acute primary angle closure cases and normal cases. They postulated that this difference might be associated with relatively anteriorly positioned ciliary processes. As in Yeung et al's study, the features in our patients also matched the plateau iris configuration described by Ritch24 and Pavlin et al9 in which the ciliary process, situated anteriorly, provides structural support beneath the peripheral iris and pushes the iris up against the trabecular meshwork. This lends support to the hypothesis that the anterior placement of ciliary process is a predisposing factor to the development of PAS.
The formation of PAS cannot be explained solely by a shorter TCPD. It is rather a multi-mechanism process resulting from the interplay between various predisposing factors such as high IOP, iris stickiness, iris contour, and other unrevealed factors. In our results, 77.8%(7/9) of the eyes had PAS in superior or inferior quadrant, and 44.4%(4/9) had PAS in the superior quadrant. Documentation of the shorter TCPD in the superior quadrant, the most common site of occlusion, provides additional support to the hypothesis that anterior placement of the ciliary process is a predisposing factor to the development of PAS. However, further studies will be needed to clarify whether other factors such as gravity contribute to the formation of PAS.
There are some limitations in this study. In some patients, both eyes were enrolled. As this was initially designed as a pilot study, we included both eyes from the same patient. Unlike IOPs seen in the general population, a high proportion of asymmetry of clinical characteristics has been reported in angle-closure glaucoma patients.25 Nevertheless, it is still a possibility that inclusion of both eyes from a single patient may have influenced the results. Therefore, further studies with a larger number of cases which includes only one eye from each subject are necessary to rule out the possibility of a selection bias. Another limitation is that this study is a retrospective cross-sectional one. As the UBM angle parameters before PAS formation are not available, it is not clear whether the shorter TCPD led to the PAS formation or vice versa. Clarification of this issue awaits a longitudinal prospective study.
In conclusion, AOD500, TIA and ARA, representing a relationship between the trabecular meshwork and the iris, were generally narrower in the superior and inferior angles in PAS-positive eyes than in PAS-negative eyes, although the differences were not statistically significant. However, the TCPD was significantly smaller in the superior quadrant in the eyes with PAS than those without PAS. We believe that the shorter distance from the trabecular meshwork to the ciliary body or anterior placement of the ciliary process may play a role in the development of PAS in angle-closure glaucoma suspect.
Notes
* This study was presented at the 95th annual meeting of the Korean Ophthalmological Society in April, 2006.

