Clinical Characteristics and Long-term Outcomes in Diabetic Macular Edema Patients: With or without Additional Injection Following Ranibizumab Loading
Article information
Abstract
Purpose
To investigate the baseline characteristics in patients with diabetic macular edema (DME) during 7 years according to the need for additional treatments after intravitreal ranibizumab (RBZ) loading injections.
Methods
The medical records of 32 patients treated with intravitreal RBZ loading for DME during 7 years were reviewed. After three-consecutive monthly RBZ injections, additional treatment was decided according to the patient’s response to RBZ loading. Based on whether the patients received treatment with or without additional injections, including intravitreal antivascular endothelial factor or steroid injection, they were divided into the “no add (NA)” or “add” groups, respectively. The baseline best-corrected visual acuity (BCVA), macular volume (MV), central subfoveal thickness, and other clinical factors were analyzed, and their 7-year changes were compared between the two groups.
Results
The BCVA of the NA group was better than that of the add group at 2, 3, 5, and 7 years (year 2, 3, and 5; p < 0.01, respectively). Baseline MV was significantly smaller in the NA group than in the add group (10.72 ± 0.88 μm vs. 11.98 ± 1.64 μm, p = 0.008). The DME duration before treatment in the NA group was significantly shorter than in the add group (1.03 ± 0.98 years vs. 1.91 ± 1.33 years, p = 0.042). The proportion of patients with serous retinal detachment or cystic macular edema was significantly lower in the NA group than in the add group (35.3% vs. 73.3%, p = 0.042). The NA group had smaller MV until 2 years than the add group (year 1, p = 0.002; year 2, p = 0.006).
Conclusions
The DME patients without additional treatments during 7 years after the initial loading treatment had shorter duration of DME and diffuse retinal thickening morphologic type with lower MV at baseline, and better long-term visual prognosis.
Diabetic macular edema (DME) is a major complication of diabetic retinopathy (DR) that causes vision impairment in adults [1,2]. The pathogenesis of DME is complex, comprising several factors resulting from the destruction of the inner hematopoietic barrier of the macula, which leads to plasma and fluid leakage from the capillaries into and out of the retinal cells [3,4].
Intravitreal antivascular endothelial growth factor (anti-VEGF) therapy is currently the first-line treatment for DME. Several studies have reported that initial intravitreal anti-VEGF injections in patients maintained the visual acuity and retinal structures [5–7]. The safety and efficacy of anti-VEGF therapy in DME patients have been evaluated in several clinical trials [8–10]. Analysis of the RIDE/RISE studies showed that monthly intravitreal ranibizumab (RBZ) injections (Lucentis; Genentech Inc., San Francisco, CA, USA) after 1 year or 3 years maintained the vision gain with a marked reduction in treatment frequency. Additionally, some patients did not require any additional treatment during the follow-up. Randomized trials on the treatment of DME with RBZ provide strong long-term evidence that it is effective in reducing the DR severity and inhibiting disease progression [6,11]. According to the VISTA/VIVID extension study, significantly greater functional and anatomical improvements were achieved in the intravitreal aflibercept injection (Eylea, Regeneron Pharmacuticals Inc., Tarrytown, NY, USA) group than in the laser group during long-term follow-up. Moreover, the efficacy was similar with both low and high frequencies of intravitreal af libercept injection administration, which could reduce the treatment burden on DME patients [12].
Several studies demonstrated that the baseline DME types according to optical coherence tomography (OCT) patterns might be predictive factor of prognosis after RBZ injections [13–15]. The frequency of additional injections during the 36-month follow-up was higher in patients with serious retinal detachment (SRD) or cystic macular edema (CME) types of DME. Notably, diffuse retinal thickening (DRT) type of DME maintained a good response to RBZ loading and needed less number of additional injections [14,15].
In this study, a 7-year follow-up was performed for patients with DME after intravitreal RBZ loading to compare patients without additional injection (no add, NA group) and those with additional anti-VEGF or steroid injection (add group). The main outcomes measured were best-corrected visual acuity (BCVA), macular volume (MV) and central subfoveal thickness (CST). Most previous studies involved a 36-month follow-up, and there are only few reports with longer follow-up periods. This is the first 7-year follow-up study of the pro re nata treatment after RBZ loading that compares the clinical characteristics and long-term outcomes of patients with and without additional injections.
Materials and Methods
Ethics statement
This study was conducted in accordance with the principles of the Declaration of Helsinki. The Institutional Review Board of KyungHee University Hospital approved the protocol (No. 2021-05-005). The need for informed consent was waived due to the minimal risk of divulging personal information.
Study design and data analysis
This retrospective study examined 32 patients who were followed up for more than 7 years from a previous prospective case-matched comparison study at Kyung Hee University Hospital. Previous prospective study analyzed 49 of 55 patients at the 12-month follow-up with three-consecutive monthly initial RBZ loading. The medical records were analyzed retrospectively for more than 6 years. During that, additional treatment was decided according to the patient’s response to RBZ loading and recurrence of DME. The baseline variables included sex, age, duration of diabetes mellitus (DM) and DME, insulin injections, glycated hemoglobin (HbA1c) level, and BCVA, which were collected from the medical records from November 2011 to October 2021. The duration of DM was defined as the duration between diagnosis of DM and initiation of RBZ loading. The DME duration before treatment was defined as the duration between the first clinically diagnosed retinal thickening or intraretinal cyst in the posterior pole (DME signs) and initiation of RBZ loading.
In this study, 32 eyes with DME were classified into two groups, depending on whether additional injections were administered. The CST and MV measurements and DME classification were performed using Cirrus HD-OCT macular cube (512 × 128 scan mode; Carl Zeiss Meditec, Dublin, CA, USA). The DME classification according to the morphologic type in this study was based on the type that had been previously classified [15]. OCT images were read by two retinal specialists in a masked manner (Kappa value, 0.902). Disagreements of classification between the examiners were resolved through discussion. DRT was defined as the presence of a sponge-like retinal thickening of the macula with intraretinal hyporeflectivity. CME was defined by the presence of highly reflective septa separating and low reflectivity cystoid-like cavities in the macular area but excluded in the presence of subretinal fluid (SRF). SRD was defined as the presence of SRF between the retina and retinal pigment epithelium, and it included the cases with DRT and CME types (Fig. 1A–1C) [14,15].
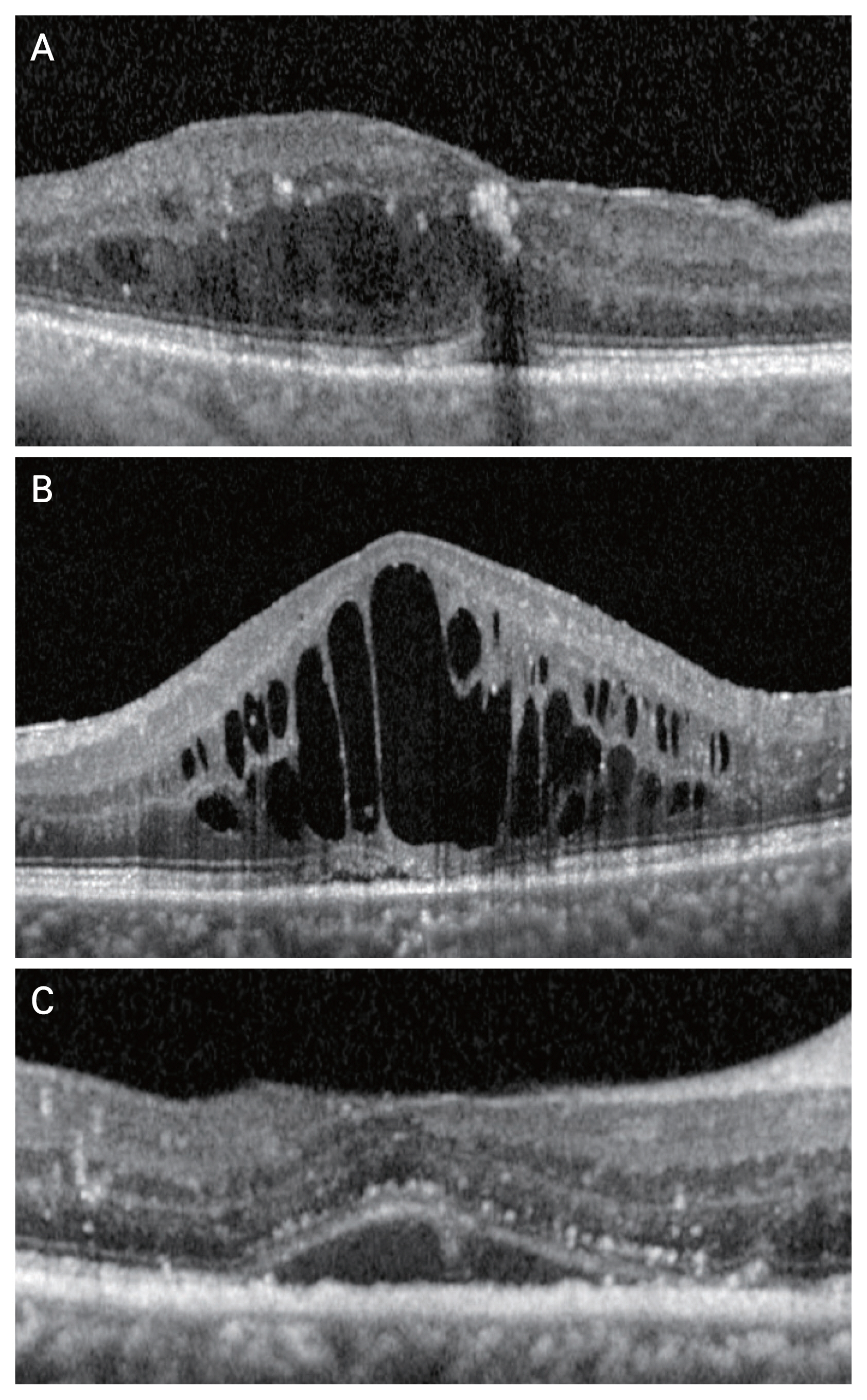
Diabetic macular edema based on the morphologic pattern on optical coherence tomography. (A) Diffuse retinal thickening type presents as a sponge-like swelling area with reduced retinal reflectivity. (B) Cystoid macular edema type shows intraretinal cystoid space. (C) Serous retinal detachment exhibits elevation of the retina and fluid is accumulated between the retina and retinal pigment epithelium.
Study participants and 7-year follow-up
The inclusion criteria of patients enrolled in the previous study were age >18 years, vision loss caused by macular edema (ME) after DR diagnosis due to type 1 or 2 DM, BCVA between 20 / 160 and 20 / 32 on the Snellen eye chart and corresponding 39 and 78 characters of visual acuity of the Early Treatment Diabetic Retinopathy Study chart, and ≥300 μm thickness of the central macula on OCT. The exclusion criteria were presence of active endophthalmitis or intraocular infection during the follow-up period, uncontrolled glaucoma despite treatment, vitrectomy or scleral buckling performed after initial intravitreal injections, history of stroke with or without visual impairment, and untreated or posttreatment systolic blood pressure of 160 mmHg or diastolic blood pressure ≥100 mmHg.
After DME diagnosis, intravitreal RBZ injection (0.5 mg) was administered three times consecutively at 1-month intervals. An additional intravitreal anti-VEGF or steroid injection was performed when one or more of the following criteria were fulfilled during follow-up: (1) a decrease in BCVA by one line or more on the Snellen chart (equivalent to ≥5 letters score) compared to last visit, (2) increased CST more than 100 μm on OCT compared to last visit or ≥300 μm thickness of the central macula, or (3) a decrease in BCVA due to newly formed intraretinal cyst or subretinal fluid, in the opinion of the retinal specialists. Additional anti-VEGF injections included bevacizumab (Avastin; Genetech, South San Francisco, CA, USA), RBZ, and aflibercept. Furthermore, the treatment was discontinued if at least one criterion was f ulfilled of the following: no improvement in the BCVA in the last three or more consecutive visits despite intravitreal RBZ injections in the last two visits and BCVA ≥20 / 20 on the eye chart in the last two consecutive visits.
Statistical analysis
The repeated measures-analysis of variance method was used to compare the BCVA, MV, and CST at baseline and at each follow-up visit over the 7-year period, and a post hoc analysis was performed using the Bonferroni method. Comparisons of the parameters between two groups at baseline were performed using the Mann-Whitney U-test for continuous variables and Fisher exact test for categorical variables. All BCVA values were converted to the logarithm of the minimum angle of resolution scale before data analysis. Statistical analyses were performed using IBM SPSS ver. 23.0 (IBM Corp., Armonk, NY, USA). A p-value of less than 0.05 was considered statistically significant. In the post hoc analysis between the two groups, significance was confirmed based on the more stringent criteria (p < 0.01) of the Bonferroni method.
Results
This study included 32 eyes of 32 patients who were followed up for over 7 years. Additional injection was not administered in 17 eyes (NA group), whereas 15 eyes received additional anti-VEGF or steroid injection (add group). The baseline characteristics are shown in Table 1. In the add group (15 eyes), a total of 102 additional injections were performed for 7 years after the initial loading, and an average of 6.8 ± 3.9 additional injections was performed in each patient. The patients visited outpatient departments 14.8 ± 1.5 times in the 1st year. The number of annual outpatient visits has gradually decreased from year 2 to year 7 (6.7 ± 3.7, 6.1 ± 4.9, 4.4 ± 2.7, 5.1 ± 2.8, 4.3 ± 2.4, and 3.6 ± 1.5, respectively). There were no significant differences between the groups in terms of their age, HbA1c levels, duration of DM, and severity of DR. The biomarkers of renal function such as estimated glomerular filtration rate, creatinine and blood urea nitrate were not significantly different. The baseline BCVA and CST were not significantly different between the groups (p = 0.582 and p = 0.063, respectively). The DME duration before treatment was significantly shorter in the NA group than in the add group (1.03 ± 0.98 years vs. 1.91 ± 1.33 years, p = 0.042). In add group (15 patients), the DME firstly recurred after an average of 8.1 ± 11.8 months. There was no significant correlation between the timing of first recurrence after loading and the initial MV or duration of DME. The proportion of patients with SRD and CME type were significantly lower in the NA group (n = 6, 35.2%) than in the add group (n = 11, 73.3%; p = 0.042).
The BCVA was compared between the baseline and 1-, 2-, 3-, 5-, and 7-year follow-up. In the NA group, the BCVA improved after loading injections and maintained the improvement until the end of the follow-up. There was a significant difference in prognosis of the two groups (p = 0.042). In comparison with the NA group, the add group showed poor visual prognosis. Two years after administration of the loading injection, the tendency to maintain the BCVA was significantly better in the NA group than in the add group at the 2-, 3-, and 5-year follow-ups (p = 0.002, p = 0.002, and p = 0.009, respectively) (Fig. 2 and Table 2).

Change in the mean best-corrected visual acuity (BCVA) during the 7-year follow-up period. In the no add (NA) group, the BCVA improved significantly after the loading injection. However, in the add group, the BCVA tended to recover in the 1st year but gradually decreased from the 2nd to 7th years. The NA group maintained better visual acuity than the add group did, especially showing a significant improvement after the 2nd year. logMAR = logarithm of the minimum angle of resolution.
The baseline MV value was significantly smaller in the NA group than in the add group (10.72 ± 0.88 mm3 vs. 11.98 ± 1.64 mm3, p = 0.008), and this significant difference was seen in the 1- and 2-year follow-ups (p = 0.002 and p = 0.006, respectively). The MV gradually decreased during the 7 years in both the groups, and there was a significant difference during the follow-up (p = 0.023) (Fig. 3 and Table 2).
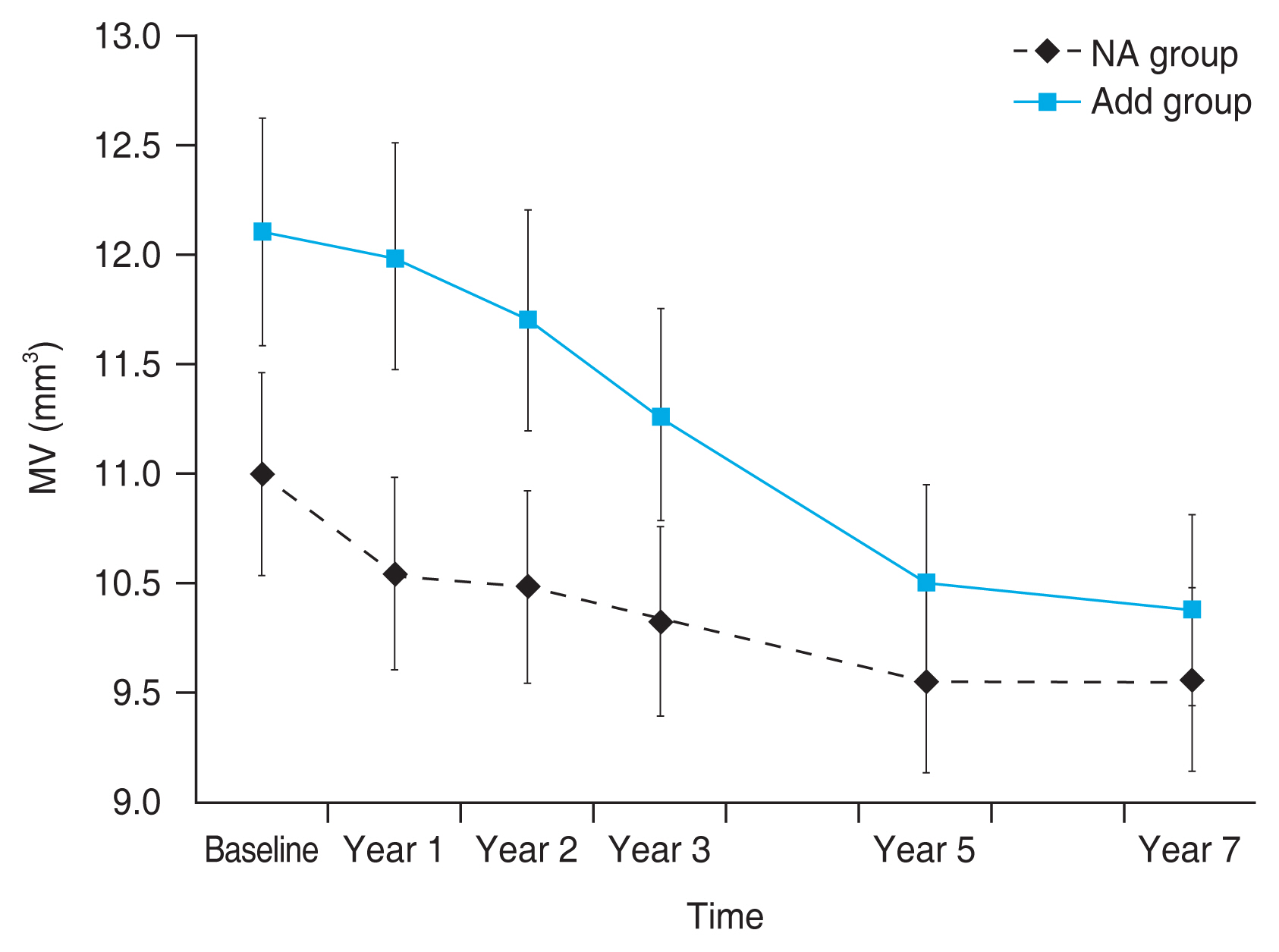
Change in the mean macular volume (MV) during the 7-year follow-up period. Compared to the no add (NA) group, the add group showed a significant difference in the MV until the 2nd year. A smaller MV was maintained in the NA group than in the add group during the 7 years.
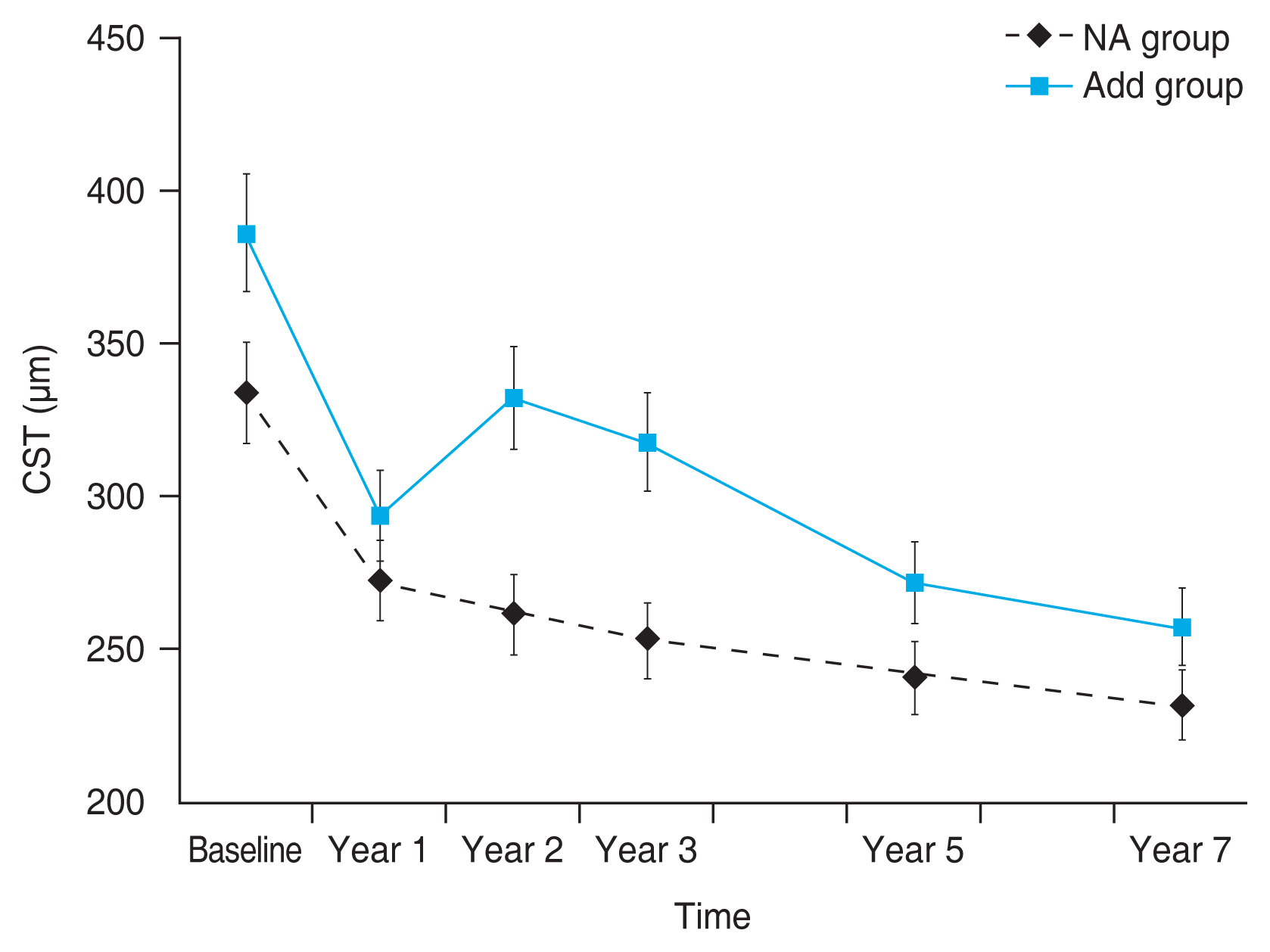
During the 7 years, the CST showed a tendency to decrease, but no significant difference was found between the two groups. The initial CST in the NA and add groups was 325.71 ± 44.77 and 386.28 ± 106.79 μm, respectively. The CST in the add group tended to decrease over the 7 years. The CST was thinner in the NA group than in the add group during the 7 years (Fig. 4 and Table 2).
Discussion
This study investigated the differences in the baseline clinical factors and long-term outcomes between patients with or without an additional injection after initial RBZ loading in DME. We found that the baseline BCVA of the NA group was better than that of the add group, and the MV was lower. In addition, the DME duration before treatment was significantly shorter in the NA group, and the percentage of SRD or CME type was significantly lower than that in the add group. Patients without additional injection showed good response to initial RBZ loading and maintained good prognosis after ME improved. BCVA improvement in the NA group was maintained from year 2 to year 7 of the study. Interestingly, the NA group showed better BCVA prognosis than the add group, even though they did not receive additional treatment after RBZ loading.
The pathophysiology of DME involves capillary dilatation and retinal microaneurysms, accompanied by damage to the blood-retinal barrier. When the blood-retinal barrier breaks down, fluid leaks into the extracellular space and disrupts the retinal cellular structure and macular function [1,16]. Structural and functional damage of the macula caused by DME is a major cause of vision loss. Several studies have reported the visual prognosis after intravitreal RBZ injection in DME patients [17–21]. Patients with good response to RBZ loading showed a better visual prognosis, which might be associated with less anatomical damages. It is worth mentioning that patients not requiring additional injections due to the beneficial effects of initial RBZ loading were expected to have better BCVA in the long-term follow-up.
Compared with the add group, the baseline MV of the NA group was significantly smaller, which was expected to be related to less severe ME. In addition, our study showed significant differences in the anatomical outcomes of MV in the early stages of the study. MV showed a decreasing trend over the follow-up period in both groups and significantly smaller MV in the NA group than in the add group until the 2nd year of the study. Patients who had better response to the initial RBZ loading had ME that improved more rapidly and maintained better retinal structure outcomes. These results corroborated the findings of several previous studies that demonstrated a reduction in the retinal thickness after anti-VEGF injections [9,22,23].
Another interesting finding of this study is that the DME duration before treatment was significantly shorter in the NA group than in the add group (p = 0.042). According to previous reports, the continuation of the hyperglycemic state due to DM triggers metabolic processes such as the de novo synthesis pathway, diacylglycerol-protein kinase C pathway, and synthesis pathway of free radicals and advanced glycation end products, eventually leading to DR. There are additional inflammatory mechanisms causing f luid leakage into the extracellular space, resulting in DME, which in turn damages the microvascular structure in the retinal layer [24]. Thus, it is thought that a long duration of DME causes structural damage to the retinal layer. Ip et al. [25] reported that the initial injection in the early course of DME maximized the treatment benefits and delaying DME treatment with RBZ injection decreased the chances of preventing progression of the disease as well as reducing DR severity. Therefore, it is possible to reduce the retinal structural damage due to DME if initial loading is performed earlier after DME diagnosis.
In this study, 73.3% of the add group and 35.2% of the NA group showed SRF or intraretinal cystic changes, which are observed in the SRD or CME types of DME, respectively. In previous reports, more than 80% cases of the DRT type of DME maintained a good response to RBZ loading without additional injections for 36 months, as compared to 33.3% cases of the CME type and 50.0% cases of the SRD type [14,15]. These studies showed that the DRT type responded better to the initial RBZ loading compared to the SRD or CME type. Thus, it is speculated that eyes with the DRT type of DME at baseline would be less likely to receive additional injections than those with the SRD or CME types, during the long-term follow-up. These data suggests that SRD or CME types of patients are expected to require more careful follow-up, considering the possibility of additional injections.
It was previously reported that in patients with ME, the HbA1c level was the most significant factor affecting ME, suggesting that DM control is important for a favorable treatment response [25]. Moreover, previous study showed a correlation between the CST and HbA1c levels in patients with DR [26]. Other study was reported that the average HbA1c and the variability of HbA1c level were not significantly related to the clinical outcomes after intravitreal anti-VEGF injection [27]. In this study, the baseline HbA1c levels were not significantly different between the NA and add groups (p = 0.159); however, the latter showed a slightly worse glycemic control than the former (8.67% ± 1.68% vs. 7.59% ± 1.02%). Further studies are warranted to investigate the correlation between glycemic control and reducing the possibility of additional injections.
The limitations of this study include its retrospective design which was a barrier to control the types of anti-VEGF or steroid drug in the add group. In this study, 23 patients were lost to follow-up before the 7-year period, which may have led to a selection bias. The small sample size may not be representative of all DME patients who underwent RBZ loading, and may limit the reliability of the results and unverified statistical normality when comparing the groups. Future research should address some of these limitations by incorporating a larger sample. Despite the limitations, the strength of this study was that it is the first study to compare the 7-year follow-up after initial RBZ loading in DME with and without additional injection.
In conclusion, the DME patients without additional treatments during 7 years after the initial RBZ loading treatment had shorter duration of DME. The SRD and CME type at baseline may require additional injections due to DME recurrences and be considered for closer clinical monitoring to maintain good prognosis.
Acknowledgements
None.
Notes
Conflicts of Interest: None.
Funding: None.