Thermal Injury Induces Small Heat Shock Protein in the Optic Nerve Head In Vivo
Article information
Abstract
Purpose
To investigate the induction pattern of various heat shock protein (HSP) in the optic nerve head after thermal stress using transpupillary thermotherapy and to determine the dose-response relationship of thermal stress on the induction of various HSP.
Methods
The 810-nm diode laser with 50-μm spot size was aimed to the center of optic nerve head of right eye of Norway brown rats. First, the various exposure powers (100, 120, 140 mW) were used with the same exposure duration, 60 seconds, to investigate power dosing effect. Second, the various exposure durations (1, 2, 3, and 5 minutes) were applied under constant 100 mW laser power to investigate time dosing effect. Left eyes were served as controls. To quantify HSP expression, enucleation was performed at 24 hours after transpupillary thermotherapy. HSP 27 and αB-crystallin inductions in optic nerve head were examined with Western blot.
Results
All type of HSP was observed in normal state. After thermal injury, the expression of HSP 27 were increased, and the αB-crystallin were decreased.
Conclusions
Induction pattern of each HSP in the optic nerve head were different after thermal injury. Some HSPs were induced or exhausted. Further research is needed on the characteristic functions and induction conditions of each HSP.
Heat shock proteins (HSPs), intracellular molecular chaperones, are a superfamily of stress proteins and exist in various states in almost all living cells [1]. Under environmental stress, these proteins are induced to protect cells and are known to enhance the survival of damaged cells [2,3]. HSPs can be classified according to molecular weight and play a critical role in protein homeostasis against noxious stimuli by assisting in protein folding, assembly, and repair together with cytoskeletal organization. Several factors such as heat, cold, ischemia, and viral infection are known stimuli that induce HSPs [4–7].
Many studies have shown that HSPs are induced by subthreshold preconditioning, and that induced HSPs show a cytoprotective effect against other insults. To make use of the cytoprotective effect of HSPs in the optic nerve to treat diseases such as glaucoma, induction of these proteins has been explored in various ways [8,9]. Previous studies have reported that induction of HSP70 in the mammalian central nervous system by hyperthermia is linked to neuronal tolerance against ischemic insults and to a neuroprotective effect against light-induced injury in the rat retina [10–12].
Various HSPs exist in normal conditions, and the optimal state for induction varies by HSP. It has been reported that the type of induced HSP can be different even within the same tissue [13]. Elsewhere, the atrial tissue of patients with persistent atrial fibrillation showed exhaustion of HSP27 and HSP70 but not of HSP40 and HSP90 in comparison with control patients in sinus rhythm [14,15]. Given this evidence, the accumulation of knowledge concerning the pattern of induction of each HSP and the variation in threshold of stress dosing with each HSP induction may be important to understand the neuroprotective mechanism in each neural tissue to overcome intractable neurodegenerative disease. However, most studies on HSPs in the optic nerve have focused on HSP70. In our previous research, transpupillary thermotherapy (TTT) with laser was proposed as a method for stably inducing HSP70; we found that TTT can stably induce HSP70, which protects the optic nerve [9,16]. As such, the aim of the present study was to investigate various HSP induction patterns in the optic nerve head after thermal injury using TTT and to discern the dosing effect of each HSP induction.
Materials and Methods
All experimental procedures were designed to conform to the Association for Research in Vision and Ophthalmology Statements on the Use of Animals in Ophthalmic and Vision Research and to Seoul National University Hospital’s guidelines. Eight-week-old Norwegian brown rats weighing between 200 and 250 g were used in the experiments. Preoperative ocular examinations were conducted under deep anesthesia with intramuscular injections of a cocktail of ketamine and xylazine. Pupil dilatation was achieved with 0.5% tropicamide and 10% phenylephrine eye drops. Cover glass was placed with viscoelastics on the cornea to view the retina. Slit lamp (BM 900; Haag-Streit, Bern, Switzerland) biomicroscopic examinations were performed before TTT to exclude other ocular abnormalities in both the anterior and posterior segments. After completing all processes, the animals were sacrificed, and their eyes were enucleated.
TTT
An 810-nm diode laser (Iridex; Quantel-Medical, Clermont-Ferrand, France) with a TTT adaptor was installed on the slit lamp (BM 900) and used in continuous mode. The rats were anesthetized deeply with the same cocktail solution and cover glass was gain placed on the cornea with viscoelastics. Subsequently, TTT was performed on right eyes, wherein a laser beam was focused on the center of the optic nerve head with a spot diameter of 0.5 mm.
First, various exposure powers (100, 120, and 140 mW) were applied for the same exposure duration (60 seconds) to reveal the power-dose response. Then, as we previously confirmed that 100 mW-induced HSPs did not produce gross optic nerve head tissue damage [9], we applied this power level for exposure durations of 1, 2, 3, and 5 minutes to assess the time-dose response. Left eyes were used as controls. We performed western blotting to quantify the induced HSPs (i.e., 27, 47, 90, and αB-crystallin).
Western blot analysis
To quantify HSP expression in the optic nerve head, western blot analysis was performed using HSP-specific antibodies and β-actin as the internal control. Twenty-four hours after TTT, which was deemed the maximum expression time [9], eyeballs were enucleated, and optic nerve heads were dissected. The optic disc of rat is small, so the minimum amount of protein required to perform the western blot is about three eyes. We performed five experiments per group. Therefore, it was set as the average amount after five eyes. The proteins from optic nerve head extracts were separated on a sodium dodecyl sulfate-polyacrylamide gel and transferred to nitrocellulose membranes. The membranes were incubated with 0.1% Tween-20 in Tris-buffered saline containing 5% nonfat dried milk for 1 hour, with mouse monoclonal antibodies specific to each HSP (i.e., 27, 47, 90 and αB-crystallin, 1:1,000; StressGen Biotechnologies, San Diego, CA, USA) for 3 hours, and with goat anti-mouse immunoglobulin G (1:5,000; Santa Cruz Biotechnology, Dallas, TX, USA) for 1 hour. The immunoreactive bands were detected using an enhanced chemiluminescence kit (Amersham Pharmacia Biotech, Little Chalfont, UK). Quantitative values were obtained by densitometry.
Results
All types of HSP were observed in the normal state (Fig. 1–4). After thermal injury with TTT, HSP27, and HSP90 were expressed more strongly in the treatment group than in the control group, while the expression levels of HSP47 and HSP αB-crystallin were decreased. When changing the power (Fig. 1, 3A, 3B), the increased expression levels of HSP27 were maintained. However, the decreased expression levels of HSP αB-crystallin observed following TTT with 100 mW of energy for 1 minute were increased to the level of control with increasing laser power. Upon changing the exposure duration (Fig. 2, 4A, 4B), the expression levels of HSP27 were similarly increased and maintained, while those of HSP αB-crystallin were reduced relative to the control values following longer application of TTT for 1 to 2 minutes.

Heat shock protein (HSP) induction by variable degrees of laser power for a constant length of exposure time (1 minute). All types of HSPs were observed in the normal state. After thermal injury with transpupillary thermotherapy, HSP27 was expressed more strongly than in the control state, while the expression levels of αB-crystallin was decreased.
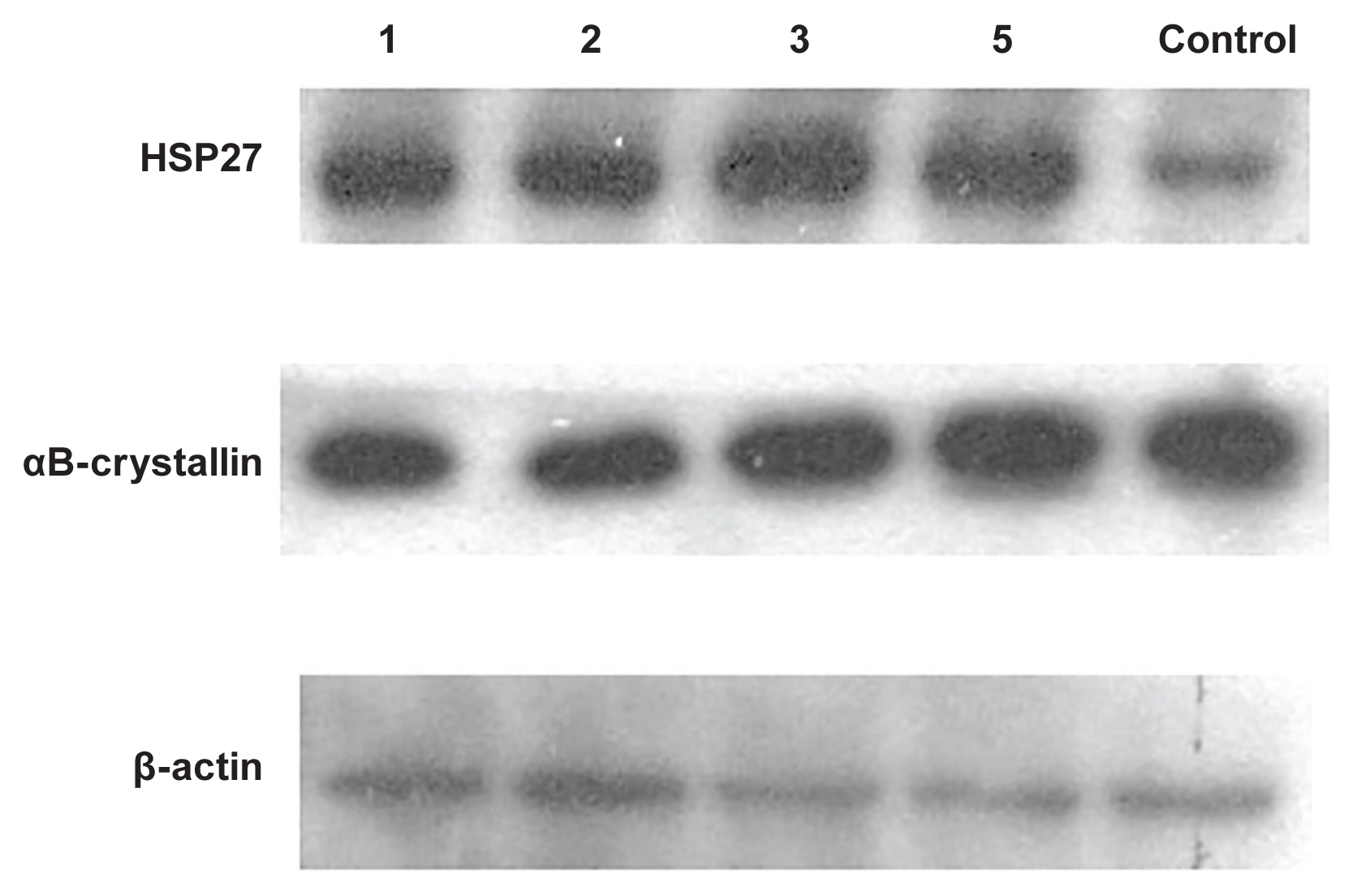
Heat shock protein (HSP) induction by constant power (100 mW) for variable laser exposure durations. All types of HSPs were observed in the normal state. After thermal injury with transpupillary thermotherapy, HSP27 was expressed more strongly than in the control state; meanwhile, the expression levels of αB-crystallin was decreased following transpupillary thermotherapy.
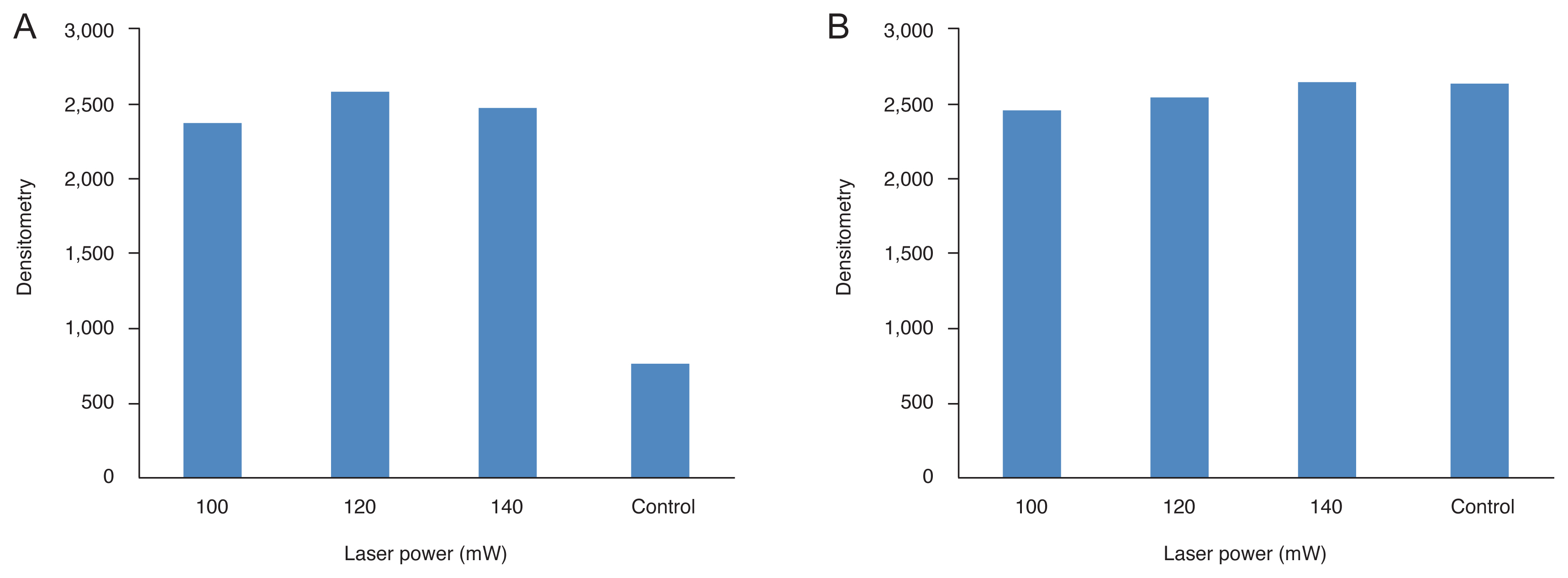
Densitometry values of heat shock proteins (HSPs) induced by variable laser powers for a constant period of exposure (1 minute). All types of HSPs were observed in the normal state. After thermal injury with transpupillary thermotherapy (TTT), (A) HSP27 was expressed more strongly than in the control state. Upon changing the power, HSP was induced more strongly in TTT eyes than in untreated control eyes, but differences in HSP expression levels at variable power levels were not distinct. Meanwhile, the expression levels of both (B) αB-crystallin was decreased after TTT.
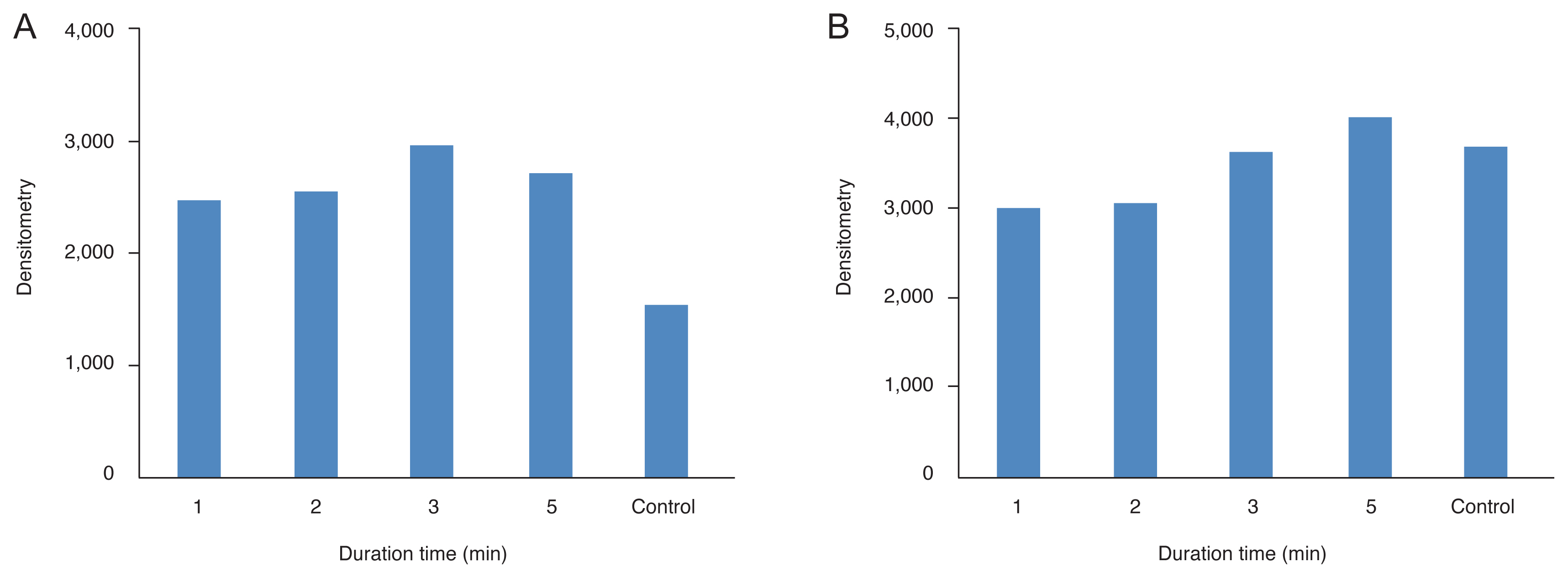
Densitometry values of heat shock proteins (HSPs) induced by constant power (100 mW) for variable laser exposure durations. All types of HSP were observed in the normal state. After thermal injury with transpupillary thermotherapy (TTT), (A) HSP27 was expressed more strongly than in the control state. Upon changing the exposure duration, HSP27 was induced more strongly in TTT eyes than in untreated control eyes, but there were no differences in expression levels of other HSPs according to exposure duration. The expression levels of (B) αB-crystallin was decreased after TTT for 1 to 2 minutes but increased to the corresponding levels in the control state after 3 minutes or more.
Discussion
HSPs are cytoprotective proteins that may play important roles in survival of the optic nerve head and its surrounding tissue under exposure to noxious stimuli [13]. HSPs are able to protect cells, protein refold, and enhance cell survival in response to various external insults. In addition, types or patterns of HSPs expressed in the same tissue may vary depending on type of insult [13]. Also, functions vary by HSP, and each can perform different functions depending on situation. It is thought that the expressed pattern and type of each HSP may differ according to function. Our results show that the patterns of individual HSP expression levels in the same tissue can differ by the amount of thermal stress. All HSPs are expressed in the normal state, probably because the optic nerve usually is subjected to a lot of light and experiences a significant degree of oxidative damage. Therefore, more than a certain amount of HSPs is always present to perform a cell-protective role against physiologic environmental insults [17]. To induce HSPs, we caused thermal injury using a TTT laser. According to our results, the expression levels of HSP27 was increased, and the expression levels of αB-crystallin was decreased after thermal injury. These variable expression patterns are thought to be due to differences in actions in response to stress or different stress thresholds of induction among HSPs. However, considering that the increased expression of HSP αB-crystallin correlated with compounded thermal injury, increased damage according to increased stress may induce the expression of almost all HSPs. And our results showed changes in the expression of HSP according to damage. However, it is difficult to prove whether these changes were intended to enhance neuroprotection of HSPs. It may simply be a change in the expression pattern according to the degree of damage.
Each HSP works independently or in concert with others to perform a cell-protective role. The cell-protective role of HSP is correlated with its chaperone functions, which are the result of its anti-apoptotic and anti-necrotic effects. HSP70 can inhibit caspase-independent cell death by interacting with apoptosis-inducing factor [18]. Overexpression of HSP70, in combination with HSP40, reduced the accumulation of abnormal polyglutamine protein and increased cell survival in various cellular models of polyglutamine diseases [19,20]. In the brain, expression of both HSP70 and HSP25 by geldanamycin could protect neurons against focal ischemia, helping to attain improved postischemic behavioral outcomes [21].
HSP27 was first identified as an estrogen-responsive protein and a shared α-crystallin domain flanked by variable N- and C-terminal sequences [22,23]. The function of HSP27 is largely regulated by phosphorylation/dephosphorylation of N-terminal serine residues, which influences the assembly of HSP27 monomers into large oligomeric complexes (200–800 kDa) [24]. Such complexes trap misfolded protein substrates to prevent their aggregation and facilitate their correct folding in conjunction with other larger HSPs such as HSP70 or HSP90. Under oxidative stress, HSP27 can act as an antioxidant, lowering the levels of reactive oxygen species by reducing the intracellular iron level and increasing the intracellular glutathione level [25]. In the retina, HSP27 may play a role as an endothelial barrier in conjunction with a general anti-apoptotic protein since downregulation of HSP27 in retinal capillary endothelial cells may lead to a vulnerable environment and increase apoptosis [26]. Elsewhere, HSP27 has been proposed as a vital protective factor in atherosclerosis [27]. Given that vascular dysregulation is a possible systemic risk factor for glaucoma, this may be an important finding by which to limit development or progression of glaucoma [28].
Mammalian crystallins include α-, β-, and γ-crystallins, with α-crystallin the main type of lens crystalline [29]. αB-crystallin can be found in the retina, cornea, optic nerve, astrocytes, and Müller cells in ocular tissues; may regulate several antiapoptotic pathways; and may have a cell-protective effect through chaperone-like activity to increase the level of cell resistance to various types of insults like diabetes, uveitis, or bacteria endophthalmitis [30].
Previous studies have reported that HSP27 and αB-crystallin contribute to the dynamics of stabilization of the cytoskeleton by maintaining the integrity and reorganization of actin filaments and intermediate filaments during stress [31,32]. Usually, both HSP27 and αB-crystallin are considered small HSPs. However, our results revealed that HSP27 and αB-crystallin had contrasting expression patterns. With changing laser power, HSP27 was induced more strongly after thermal injury, and this elevated expression level was maintained despite increasing laser power. The expression of αB-crystallin initially was decreased after thermal injury but returned to the level seen in the normal state with increasing laser power. The reason for this difference in pattern is not known between the two small HSPs with similar functions. If the protein production capacity is limited, in certain situations, HSPs with more proper properties increase first and significantly; otherwise, their limited production may be possible. Depending on the type or context of the stress, production of specific HSPs may be increased. And, in our study, we did not compare the results of the optic nerve of each rat, but showed the trend of the value compared to the results of taking the optic nerve of about 5 rats. This was an unavoidable result because the amount of protein that can be extracted from the rat’s eye was so small that western blot could not be performed with the amount extracted from each eye. And there is a risk of error in collecting five samples and comparing the results once tested. Further investigations are necessary to better understand this phenomenon.
Considering our results and those of previous studies reporting differences in expression patterns depending on stress situation, it is believed that various HSPs adopt the role of molecular chaperones independent of or complementary to each other. Given that HSP has a function to maintain cell survival, it is thought that the response pattern of each HSP is different depending on the intensity and pattern of stimulation. In our study, it is considered to be complementary that the expression of HSP 27 continues and the α-crystalline increases when HSP 27 is consumed. It seems that it is trying to continue to perform a protective role. For this reason, if we determine the characteristic expression pattern of each HSP in the optic nerve in the context of situation, guidelines by which to determine the HSPs most effective in protecting from glaucomatous optic nerve damage can be established. In our study, some HSPs were induced and some were exhausted in the optic nerve head after thermal injury. These results constitute preliminary data that may be further considered in future neuroprotective experiments in glaucoma with enhancement of the natural cytoprotective stress response. In our study, the induction pattern of each HSP in the optic nerve head differed after thermal injury, with some HSPs induced and others exhausted. The unique function of each HSP may have led to this variation.
Notes
No potential conflict of interest relevant to this article was reported.