Clinical Outcomes of Small Incision Lenticule Extraction in Myopia: Study of Vector Parameters and Corneal Aberrations
Article information
Abstract
Purpose
To investigate clinical outcomes of small incision lenticule extraction (SMILE) including vector parameters and corneal aberrations in myopic patients.
Methods
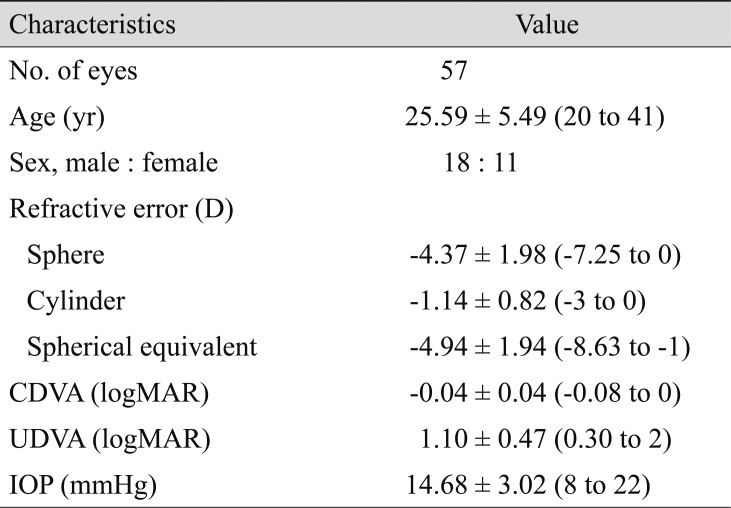
This retrospective, observational case series included 57 eyes (29 patients) that received treatment for myopia using SMILE. Visual acuity measurement, manifest refraction, slit-lamp examination, autokeratometry, corneal topography, and evaluation of corneal wavefront aberration were performed preoperatively and at 1 and 3 months after surgery. We analyzed the safety, efficacy, vector parameters, and corneal aberrations at 3 months after surgery.
Results
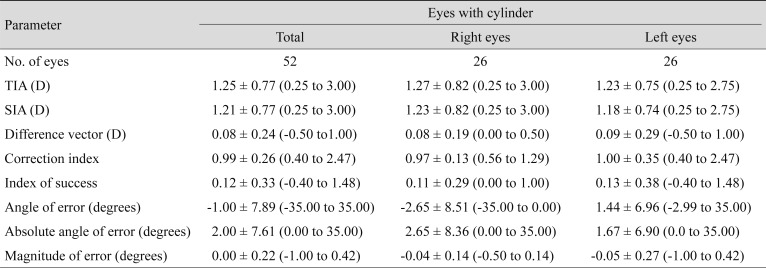
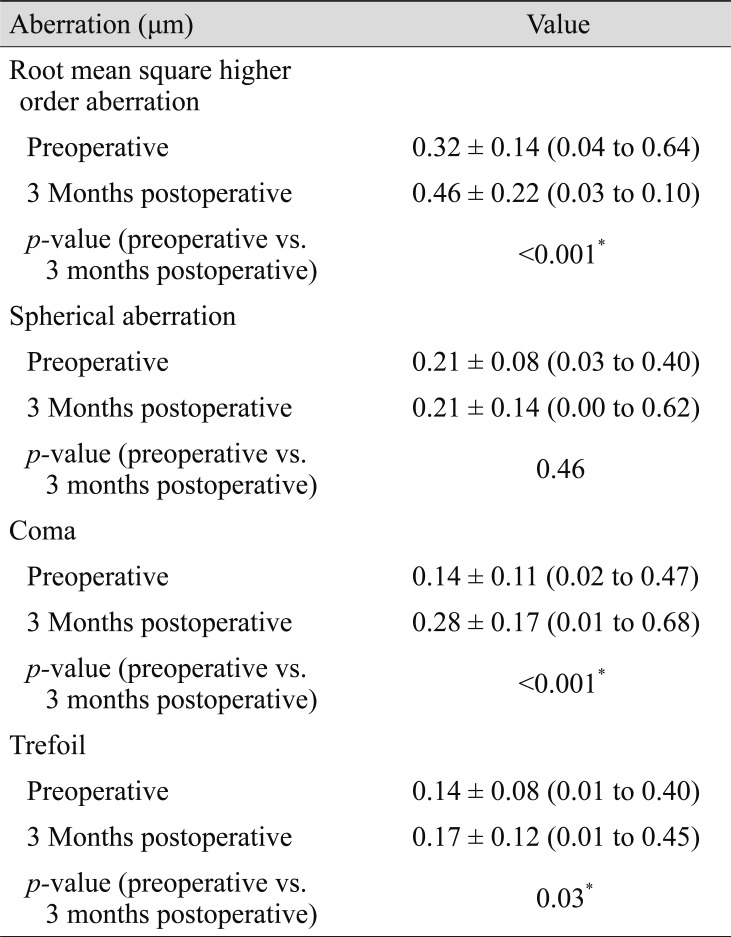
Preoperatively, mean manifest refraction spherical equivalent refraction was −4.94 ± 1.94 D (range, −8.25 to 0 diopters [D]), and the cylinder was −1.14 ± 0.82 D (range, −3 to 0 D). Mean manifest refraction spherical equivalent improved to −0.10 ± 0.23 D at 3 months postoperatively, when uncorrected distance visual acuity was 20 / 20 or better in 55 (96%) eyes. The linear regression model of target induced astigmatism vector versus surgically induced astigmatism vector exhibited slopes and coefficients (R2) of 0.9618 and 0.9748, respectively (y = 0.9618x + 0.0006, R2 = 0.9748). While total corneal root mean square higher order aberrations, coma and trefoil showed statistically significant increase, spherical aberration did not show statistically significant change after SMILE.
Conclusions
SMILE has proven to be effective and safe for correcting myopia and astigmatism. We showed that SMILE did not induce spherical aberrations. A small increase in postoperative corneal higher order aberration may be associated with increase in coma and trefoil.
Since Jose Barraquer invented keratomileusis for correction of high myopia in 1949, researchers have combined keratomileusis with excimer laser surface ablation to develop one of the most commonly used laser corneal refractive surgery techniques, laser in situ keratomileusis (LASIK) [1]. Creation of a corneal flap allows early visual recovery, less discomfort, and reduced stromal inflammation. However, this procedure can induce loss of corneal biomechanical strength and worsen dry eye [2]. Surface ablation laser surgery is another type of corneal refractive surgery. Unlike LASIK, surface ablation does not create a flap but uses an excimer laser to expose and remove the corneal stroma after removing the corneal epithelium by mechanical scraping (photorefractive keratectomy) or 20% alcohol (laser epithelial keratomileusis) [2]. Without the flap, surface ablation results in a biomechanically stronger cornea compared to that of LASIK. Technological advancements have allowed incorporation of wavefront technology to laser refractive surgery. Wavefront-guided excimer laser surgery is designed to minimize surgically induced higher order aberrations or compensate for pre-existing higher order aberrations [2]. Small incision lenticule extraction (SMILE; Carl Zeiss Meditec AG, Jena, Germany) is a relatively new flapless refractive surgery that utilizes a femtosecond laser to create a lenticule of the desired correction within the cornea, which is then extracted through a small corneal incision. The procedure was initially introduced as femtosecond lenticule extraction (FLEx, Carl Zeiss Meditec AG), which required creation of a flap similar to that in LASIK plus an additional posterior cut to create a stromal lenticule that is then extracted [3]. Surgical techniques have evolved to pseudo small-incision lenticule and small-incision lenticule extractions, which require no retractable flaps [2]. Small-incision lenticule extraction involves removal of the same lenticule through a small 2.0 to 3.0 mm pocket incision, whereas pseudo small-incision lenticule extraction is generally performed during the learning curve with a bigger pocket incision (5.0 to 6.0 mm) that allows conversion to lifting of the flap when unsuccessful [4]. Compared to LASIK, SMILE provided a similar visual outcome with a lower incidence of flap complications and dry eye [56]. As a result, SMILE is becoming a promising alternative to other refractive surgeries despite its shorter history. The objective of this retrospective study is to evaluate the clinical outcomes, including astigmatic vector parameters and corneal aberrometric changes, of SMILE in myopic patients.
Materials and Methods
Patients
This study was a retrospective, observational case series approved by the institutional review board of Yonsei University College of Medicine, Seoul, South Korea (4-2019-0521). The study followed the tenets of the Declaration of Helsinki. Written informed consent was waived due to the retrospective nature of the study. Patients enrolled in this study received treatment by SMILE from a single experienced surgeon (TK) at Severance Hospital, Yonsei University College of Medicine between January 2017 and January 2018. This study included 57 eyes of 29 subjects who underwent SMILE.
Inclusion criteria were myopia of fewer than 9.00 diopters (D), age 20 to 45 years, and corrected distance visual acuity (CDVA) of 0.7 Snellen or better. Exclusion criteria were systemic and ocular anomalies or pathologies and history of intraocular or corneal surgery.
Examination protocol
Preoperatively, all patients underwent complete ophthalmologic examination, including uncorrected distance visual acuity (UDVA) and CDVA, manifest refraction, slitlamp examination, autokeratometry, noncontact tonometry, funduscopy, and Scheimpflug-based corneal topography (Pentacam HR; Oculus Optikgeräte, Wetzlar, Germany). Corneal wavefront aberrations were measured using iTrace (Tracey Technology, Houston, TX, USA). Postoperatively, the patients were examined for UDVA and CDVA, autokeratometry, and tonometry at 1 day, 1 week, 1 month, and 3 months after surgery. We performed manifest refraction and Scheimpflug-based corneal topography at 1 week, 1 month, and 3 months after surgery. We measured corneal aberrations 3 months after surgery.
Surgical procedure
One experienced surgeon (TK) performed all surgeries with a 500-kHz VisuMax system (Carl Zeiss Meditec AG) using standardized techniques with the three-centration point marking technique [7]. After topical anesthesia with proparacaine hydrochloride (Alcaine; Alcon, Fort Worth, TX, USA), photo disruption was used to create posterior and anterior surfaces of the lenticule and side-cut opening. Following use of the femtosecond laser, the surgeon used a spatula to separate the refractive lenticule and extract it using forceps. We instructed patients to instill moxifloxacin 0.5% together with loteprednol etabonate 0.5% every two hours for the first 24 hours and then four times a day for the following two weeks. Subsequently, we tapered medications to meet each patient's condition. All surgeries were uneventful, and no complications such as suction loss, black spots, difficult dissection, incomplete separation of the lenticule, or epithelial ingrowth occurred in any eyes.
Statistical analysis
We presented the results as mean, standard deviation, and range and performed Student's t-test to evaluate the difference between preoperative and postoperative data. We compared continuous variables by linear regression analysis. Statistical analysis was performed using IBM SPSS Statistics ver. 23.0 (IBM Corp., Armonk, NY, USA). A p-value of 0.05 or less was considered statistically significant.
Results
This study included a total of 57 eyes of 29 patients who underwent SMILE between January 2017 and January 2018. Table 1 shows the demographic data for the study population. Mean preoperative spherical equivalent (SE) and cylinder were −4.94 ± 1.94 D and −1.14 ± 0.82 D, respectively.
Visual acuity, efficacy, safety, and refraction
At 3 months after surgery, there was a significant improvement in mean UDVA (from 1.10 ± 0.47 to −0.03 ± 0.05 logarithm of the minimum angle of resolution). Postoperative UDVA was 20 / 20 or better in 55 (96%) eyes at 3 months (Fig. 1A–1H). CDVA was unchanged in 52 (91%) eyes at 3 months postoperatively, while 3 (5%) eyes gained one Snellen line. Mean efficacy index (ratio of postoperative UDVA to preoperative CDVA) and mean safety index (ratio of postoperative to preoperative CDVA) at 3 months were 0.97 ± 0.08 and 1.00 ± 0.06, respectively.

Visual outcomes after small incision lenticule extraction. (A) Cumulative 3-month postoperative uncorrected distance visual acuity (UDVA) and preoperative corrected distance visual acuity (CDVA). Changes in Snellen lines of (B) postoperative UDVA and (C) CDVA relative to preoperative CDVA. (D) Attempted versus achieved changes in spherical equivalent refraction (SEQ) at 3 months after surgery. (E) The accuracy of SEQ to the intended target. (F) Comparative distribution of preoperative and 3-month postoperative cylinder and (G) target induced astigmatism (TIA) versus surgically induced astigmatism (SIA) vectors at 3 months. (H) Refractive astigmatism angle of error distribution at 3 months after surgery. D = diopters.
The mean manifest refraction SE improved from −4.94 ± 1.94 to −0.10 ± 0.23 D. Spherical equivalent refraction was within ±0.50 D in 55 eyes (96%) and within ±1.00 D in 57 (100%) eyes. The linear regression model of attempted versus achieved SE had a slope and coefficient (R2) of 0.9805 and 0.9977, respectively (y = 0.9805x − 0.0002; R2 = 0.9977).
Outcomes of astigmatism correction and vector analysis
Fifty-five (96%) eyes exhibited a postoperative cylinder of 0.50 or less (Fig. 1). Table 2 shows the vector analysis of the 3-month refractive data. We analyzed astigmatism data using the methods described by Alpins [8]. The linear regression model of target induced astigmatism vector versus surgically induced astigmatism vector showed slopes and R2 of 0.9618 and 0.9748, respectively (y = 0.9618x + 0.0006, R2 = 0.9748). The angle of error histogram showed that the refractive correction was placed on the intended meridian for most eyes (94.2% within ±15°). Due to some degree of a mirror symmetric effect in the axes of astigmatism between right and left eyes, we analyzed the data of the right and left eyes separately [9]. Since analysis of the right and left eyes separately exhibited similar tendencies to the entire sample (Table 2), we presented the polar plots of target induced astigmatism, surgically induced astigmatism, difference vector, and correction index in Fig. 2A–2D for the entire sample.
Higher order aberrations
Table 3 and Fig. 3 summarize the change in corneal aberration after surgery. Total corneal root mean square higher order aberrations, coma, and trefoil exhibited statistically significant increases from 0.32 ± 0.14 to 0.46 ± 0.22 (p < 0.001), 0.14 ± 0.11 to 0.28 ± 0.17 (p < 0.001), and 0.14 ± 0.08 to 0.17 ± 0.12 (p = 0.03), respectively after surgery. In contrast, spherical aberration did not show any statistically significant change after SMILE (from 0.21 ± 0.08 to 0.21 ± 0.14, p = 0.046).
Discussion
The current study found excellent visual and refractive outcomes 3 months after SMILE in myopic patients. Refractive outcomes of SMILE from other long-term studies were comparable to ours. One study reported UDVA of 0.01 and SE of −0.375 D with regression of 0.48 D at 5 years postoperatively [10]. A different study reported visual acuity of −0.05 logarithm of the minimum angle of resolution and mean SE of −0.01 D at 3 years after SMILE [11]. Another group reported Snellen visual acuity of 99% and SE of −0.19 D at 2 years after SMILE [12]. The efficacy and safety indices were also comparable to those in previous studies [71314]. Unlike some previous studies that have reported undercorrection of SE after SMILE, our data did not show myopic residual SE [15].
Despite some concern regarding undercorrection of astigmatism after SMILE [15], recent studies have shown effective and predictable astigmatism correction in SMILE [716]. In our study, 55 (96%) eyes exhibited postoperative astigmatism of 0.50 D or less (Fig. 1). The mean correction index was 0.99, indicating an efficient and predictable outcome. In this study, 49 eyes (94.2%) exhibited angle of error values of 15° or less. The mean and mean absolute angle of error values were −1.00° and 2.00°, respectively. These findings are comparable to previous studies on SMILE [71417] and indicate safe and predictable outcomes.
Our data showed that SMILE did not induce spherical aberration. Root mean square higher order aberration, coma, and trefoil exhibited statistically significant increases after surgery. Different researchers reported varying trends of corneal aberrometric outcomes and presented different interpretations on their outcomes. A number of groups noted a statistically significant increase in coma after SMILE [181920]. Postoperative coma can increase with decentration [21]. Studies have suggested that mild decentration without a tracking system may induce a smaller increase in coma [1822]. We tried to minimize decentration using the triple centration technique [7]. However, despite our efforts, we could not eliminate induction of coma. Other studies have reported that single incisions in the SMILE procedure may cause an imbalanced corneal healing response and induce optical changes, which could increase coma postoperatively [23]. However, postoperative increase in coma was smaller in SMILE than LASEK and was similar to that of femtosecond (FS)-LASIK [2224]. Studies reported that early wound healing response of SMILE is less reactive than that of FS-LASIK [25] and that the corneal epithelial remodeling after SMILE exhibited lesser shift toward oblateness than that after FS-LASIK [24]. Such advantages may compensate for the effect of decentration of SMILE compared with that of FS-LASIK. In addition, different working patterns of the femtosecond laser in SMILE and the excimer laser in LASIK may result in different postoperative corneal tissue structures [22].
There have been various reports on the postoperative change of spherical aberration in SMILE. Many researchers have reported that spherical aberration did not show statistically significant change after SMILE [46192326]. However, patients with more severe myopia showed a statistically significant increase in spherical aberration [182027]. Such discrepancies might arise from differences in instruments, scotopic environments, and pupil size [18]. Moreover, a thicker stroma lenticule from higher myopic correction could result in a large change in anterior corneal asphericity and induce greater spherical aberration [1820]. However, many reports agree that induction of spherical aberration in SMILE is not as significant as in excimer laser surface ablation [282930]. The energy efficiency of excimer laser is not uniform from the center to periphery, thereby decreasing the ablation rate toward the periphery, which may induce higher order aberration [22]. On the other hand, a femtosecond laser can excise corneal tissue more accurately, minimizing induction of spherical aberration. Higher order aberration, including spherical aberration, was consistent between SMILE and FLEx, suggesting that higher order aberration is related more to ablation of corneal tissue and less to creation of flaps [31].
Another factor that might influence measurement of aberration of the anterior corneal surface is dry eye [18]. The influence of dry eye was minimal after SMILE due to the relatively small interference in tear film, thereby decreasing the effect of dry eye in aberration measurement in SMILE [18].
Our result is noteworthy in that, even without the wavefront-guided technique, SMILE did not induce spherical aberration, one of the major factors that cause visual disturbances such as halo and glare. Studies have shown that other corneal refractive surgeries, including LASIK and photorefractive keratectomy, induce spherical aberration [153233]. One feature in our SMILE surgical technique is the triple centration technique. We attribute our result to the advantage of using this technique, which allowed better centration and horizontal cyclotorsion control [7]. Therefore, we believe that SMILE can be as effective as other refractive surgeries in terms of astigmatism correction and corneal aberration.
Some limitations of the current study include its relatively small sample size and lack of data for a longer follow up period. Nonetheless, this study provides an accurate analysis of corneal vector parameters and corneal aberration after SMILE in Korean patients. This study may serve as the foundation for investigating the outcome of SMILE with respect to severity of myopia and astigmatism and the trend of myopic regression with long-term follow up in the future.
In conclusion, 3-month outcomes of SMILE demonstrate that this procedure is safe and effective for myopic and astigmatic corrections.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (NRF-2019R1F1A1062468).
Notes
The abstract was presented at the 119th Annual Meeting of the Korean Ophthalmological Society in Busan, Korea, April 7–8, 2018.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.