Efficacy of 4-Haptic Bitoric Intraocular Lens Implantation in Asian Patients with Cataract and Astigmatism
Article information
Abstract
Purpose
To determine the efficacy of 4-haptic bitoric intraocular lens (IOL) implantation in Asian patients with cataract and astigmatism.
Methods
A total of 19 eyes with ≤25.0 mm axial length and ≥0.75 diopters (D) corneal astigmatism were included in this prospective non-comparative study. All subjects underwent phacoemulsification with implantation of an AT Torbi 709M IOL. Visual and refractive outcomes as well as toric IOL axis were evaluated during a 3-month follow-up. Errors in predicted residual spherical equivalent were calculated by subtracting predicted residual spherical equivalent from postoperative refraction.
Results
Uncorrected and corrected distance visual acuity improved significantly 3 months after surgery, from 0.43 to 0.05 and from 0.24 to −0.05, respectively. Mean refractive cylinders also decreased significantly, from −1.91 preoperatively to −0.54 D 3 months after surgery. Mean J0 and J45 decreased 3 months postoperatively, from 0.26 to 0.03 D and from 0.24 to −0.06 D, respectively. After 3 months, mean absolute IOL rotation was 1.81°. Errors in predicted residual spherical equivalent showed a hyperopic shift of 0.35 D.
Conclusions
Implantation of 4-haptic bitoric IOL proved to be effective for correcting astigmatism in Asian eyes during cataract surgery.
Cataract surgery is regarded as a refractive procedure, from which patients expect to achieve a spectacle-free life after surgery. However, 20% to 30% of patients with cataract have corneal astigmatism ≥1.25 diopters (D), requiring correction of refractive errors with toric intraocular lens (IOL) implantation during cataract surgery [12]. A meta-analysis has shown that toric IOL implantation is effective, safe, and predictable for cataract patients with corneal astigmatism [3]. Toric IOL should be implanted and retained at the exact axial position to achieve the desired residual astigmatism (RAS). One study concluded that each degree of off-axis rotation will result in a loss of up to 3.3% of IOL cylindrical power [4]. When the axis of a toric IOL is misaligned by 30°, the preoperative astigmatism cannot be corrected [4].
Various toric IOLs have been developed to correct astigmatism. Toric IOLs have different material, asphericity, toricity distribution, morphology, overall haptic diameter, and calculation algorithms. Depending on lens types, efficacy for astigmatic correction and stability of the IOL may differ. Transitional conic toric IOL and bitoric IOL have been reported to provide superior image quality despite pupil size change and the presence of decentration [2]. The AT Torbi 709M toric IOL (Carl Zeiss Meditec AG, Jena, Germany) has a bitoric optic design to correct high astigmatism and a 4-haptic plate design to minimize IOL rotation. Some European studies have stated that the 4-haptic bitoric IOL is effective for correcting astigmatism [56]. However, efficacy or stability of the 4-haptic bitoric IOL in Asian eyes has not been reported. Considering that changes in capsular bag diameter are different between Asian eyes and European eyes immediately after cataract surgery [78], such changes might affect the efficacy and stability of 4-haptic bitoric IOL for astigmatism correction.
Therefore, the objective of this prospective non-comparative study was to evaluate the efficacy of the AT Torbi 709M toric IOL for astigmatism correction and rotational stability in Asian patients with cataract and corneal astigmatism >0.75 D.
Materials and Methods
Patients
This study followed the tenets of the Declaration of Helsinki. After receiving a thorough explanation of the procedure, risks, and possible complications of the study, all patients provided written informed consent. This study (accession number NCT02618018) was registered at http://www.clinicaltrials.gov. Clinical protocols were approved by the institutional review board of Seoul National University Hospital (1503-118-658). This prospective and non-comparative study included 20 eyes of 18 subjects who underwent phacoemulsification with AT Torbi IOL implantation at Seoul National University Hospital by one surgeon (MKK) between September 2015 and September 2016. During surgery, one subject was found to have disruption to one-quarter of the zonule and so was dropped from this study. Subsequently, a total of 19 eyes were included in the final analyses.
Inclusion criteria were patients aged 50 to 90 years with clinically significant cataract, an axial length (AL) <25.0 mm, and regular corneal astigmatism ≥0.75 D according to autokeratometry. Exclusion criteria were previous ocular surgery, systemic and ocular anomalies or pathologies that could reduce visual function or postoperative IOL stability (retinal detachment, amblyopia, glaucoma, macular degeneration, corneal disease, irregular astigmatism, and pseudoexfoliation syndrome), or unsuitability for adequate follow-up.
Examination protocol
Preoperatively, all patients underwent complete ophthalmologic examination, including uncorrected distance visual acuity (UDVA) and corrected distance visual acuity (CDVA), autorefractometry, autokeratometry, and biometry measurements such as AL and anterior chamber depth using partial coherence interferometry (IOL Master 500, Carl Zeiss Meditec AG), corneal topography (Orbscan II; Bausch and Lomb, Rochester, NY, USA), complete slit-lamp examination, noncontact tonometry, and fundoscopy. Postoperatively, patients were examined for UDVA and CDVA, autorefractometer data, and autokeratometry 1 day, 1 week, 1 month, and 3 months after surgery.
Surgical procedure
One experienced surgeon (MKK) performed all surgeries using a standard technique of sutureless coaxial phacoemulsification with the patients under topical and subtenon anesthesia. For preoperative corneal marking, the patient sat upright with the head carefully aligned on the slit lamp. The patient was asked to fixate straight ahead on a distant target with the contralateral eye. The fine horizontal bright slit beam crossed the center of the pupil, and two limbal points (at 0° and 180°) of the cornea were marked by dimpling with a Sinskey hook (Katena, Denville, NJ, USA). The implantation meridian was marked using a Mendez ring (Katena) just before surgery with the patient on the surgical bed. Coaxial phacoemulsification was performed through a 2.7-mm temporal corneal incision. The IOL was inserted into the capsular bag and rotated to align with the IOL cylinder axis with the steepest corneal axis. After removing any viscoelastics, if the axis was rotated, the IOL was repositioned with BSS PLUS irrigating solution (Alcon, Fort Worth, TX, USA). The same postoperative treatment, consisting of corticosteroids, antibiotics, and nonsteroidal anti-inflammatory eye drops, was administered to all patients. We used Z CALC (Carl Zeiss Meditec AG) to calculate IOL power and to determine the axis at which the IOL should be placed.
IOL
The AT Torbi 709M IOL used in this study is a foldable, one-piece, bitoric, monofocal, and aspheric lens. This IOL is made of a hydrophilic acrylic material with a hydrophobic surface. It has a plate haptic without angulation, a biconvex 6.0-mm optic, and an overall length of 11.0 mm. The lens is available at spherical diopters ranging from −10.0 to +32.0 with cylindrical diopters ranging from +1.0 to +12.0.
Astigmatism analysis
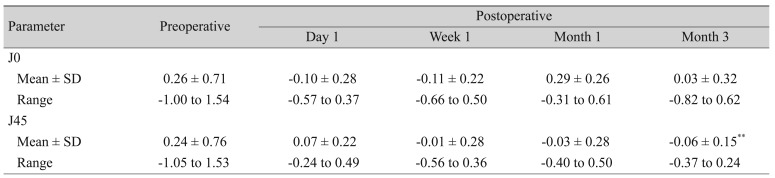
Astigmatism analysis was performed using the power vector analysis method described by Thibos and Horner [9]. J0 and J45 are two Jackson cross cylinders equivalent to a conventional cylinder with axes of 0° and 45°, respectively.
J0 = (−C/2)cos(2α)
J45 = (−C/2)sin(2α)
where C is the cylindrical power, and α is the cylinder axis.
IOL rotation analysis
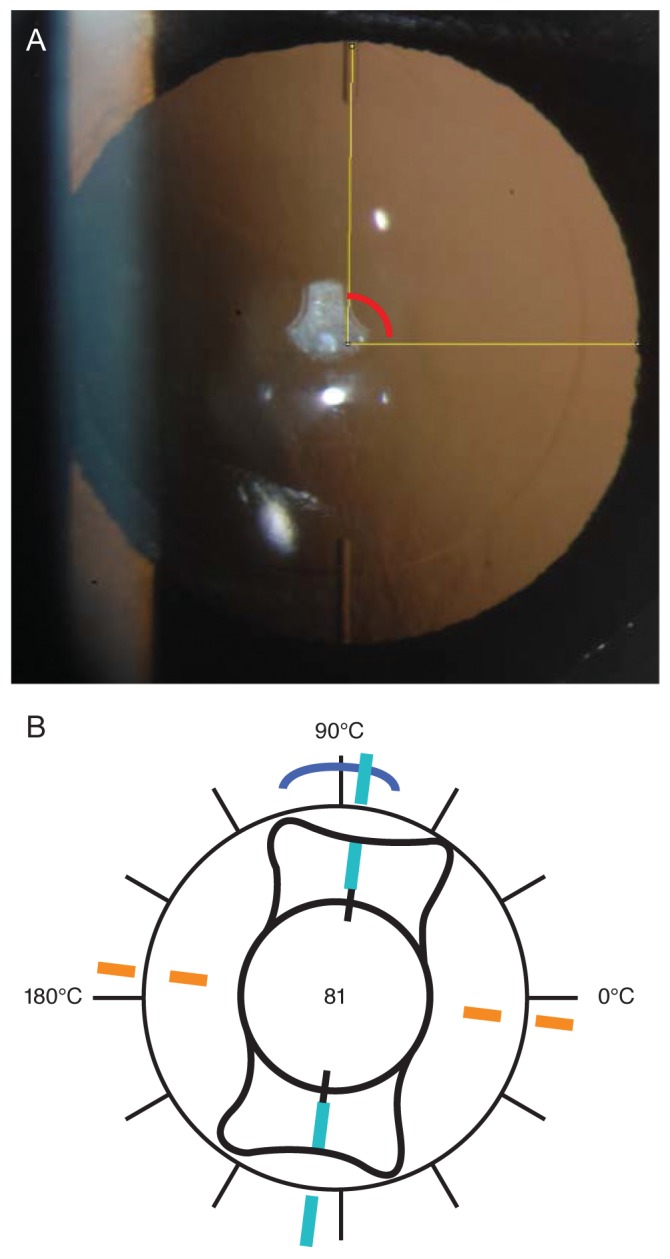
Toric IOL axis alignment was measured 1 week, 1 month, and 3 months after surgery. For our analyses, retroillumination photography with digital imaging of the IOL was performed after pupil dilation (Fig. 1A, 1B). The angle between the horizontal axis and the IOL axis was drawn using ImageJ ver. 1.48 (National Institutes of Health, Bethesda, MD, USA). Episcleral vessels were used as reference points to reduce the effects of eye and head rotation, thus improving measurement accuracy. Once a prominent episcleral vessel was identified, the axis between the vessel and the center of the IOL was drawn. In postoperative images, this axis was used to determine the horizontal axis by maintaining the angle between the episcleral vessel and the horizontal axis.
Statistical analysis
Statistical analyses were performed using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). Results are presented as mean, standard deviation, and range (minimum–maximum). We performed t-tests to evaluate the difference between preoperative and postoperative data, and we considered p ≤ 0.05 statistically significant.
Results
We evaluated 19 eyes of 17 patients with a mean age of 71.16 ± 8.52 years (range, 54 to 87 years). The IOL was implanted in the right eye of eight patients and in the left eye of 11 patients. The mean preoperative UDVA and CDVA were 0.43 ± 0.17 and 0.24 ± 0.28 logarithm of the minimum angle of resolution (logMAR), respectively. Mean corneal astigmatism was −1.78 ± 0.81 D. Mean preoperative AL and anterior chamber depth were 23.42 ± 0.62 mm (range, 21.83 to 24.18 mm) and 3.10 ± 0.35 mm (range, 2.30 to 3.94 mm), respectively. There was no iridodonesis or lens subluxation before or during cataract surgery. Demographics and preoperative data are summarized in Table 1.
Visual acuity and refraction
Visual acuity and refractive results over time are shown in Table 2. Both UDVA and CDVA improved significantly by postoperative day 1 (p = 0.002 and p = 0.013, respectively, paired t-test), and they remained stable over time. After 3 months, all eyes had better CDVA than 0.10 logMAR. UDVA was better than 0.30 logMAR in all eyes, was better than 0.10 logMAR in 15 (78.9%) eyes, and even better than 0.0 logMAR in seven (36.8%) eyes.
Statistically significant reduction was observed in manifest sphere and cylinder 1 day after surgery (p = 0.005 and p = 0.001, respectively, paired t-test). The spherical equivalent (SE) value was also reduced the day after surgery, although the reduction was not statistically significant (p = 0.062, paired t-test), possibly because preoperative SE values were very small. The manifest sphere, cylinder, and SE remained stable over time (Fig. 2A–2C). Three months after surgery, the mean RAS was −0.54 ± 0.46 D (range, 0 t o −1.75 D), which was significantly reduced (p ≤ 0.001, paired t-test) from preoperative corneal astigmatism values. RAS was within 0.50 D in 83.3% of patients and within 1.00 D in all (100%) patients.
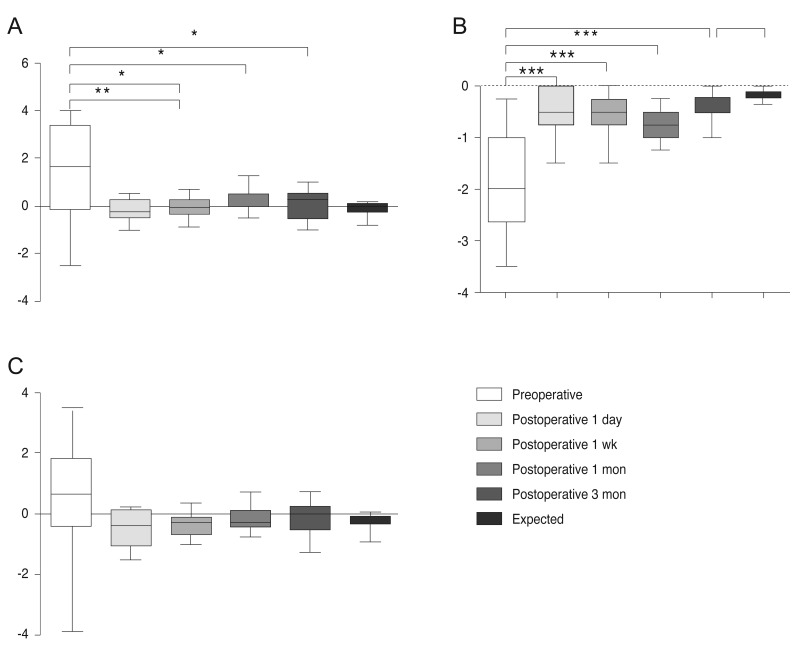
Refractive outcomes over time showing mean preoperative and postoperative values of the (A) spherical error, (B) cylindrical error, and (C) spherical equivalent. *p < 0.05, **p < 0.005, ***p < 0.0005.
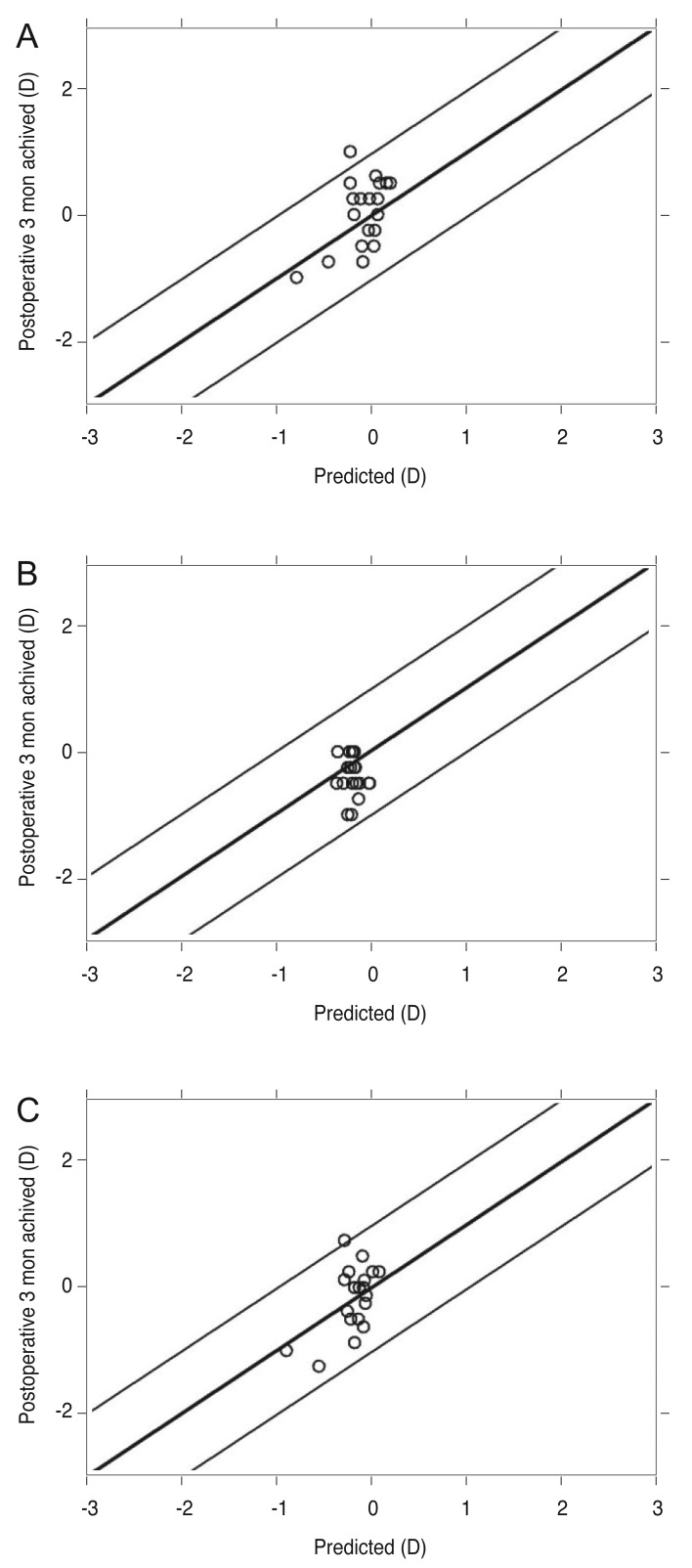
The mean differences between predicted and achieved postoperative manifest sphere, cylinder, and SE after 3 months were 0.41 ± 0.27, 0.32 ± 0.23, and 0.35 ± 0.27 D, respectively (Fig. 3A–3C). In other words, residual SE showed a hyperopic shift about 0.35 D compared with predicted SE. The preoperative and postoperative SE difference was within ±0.50 D in 14 eyes (73.7%) and within ±1.00 D in 18 eyes (94.7%).
Astigmatism vector analysis
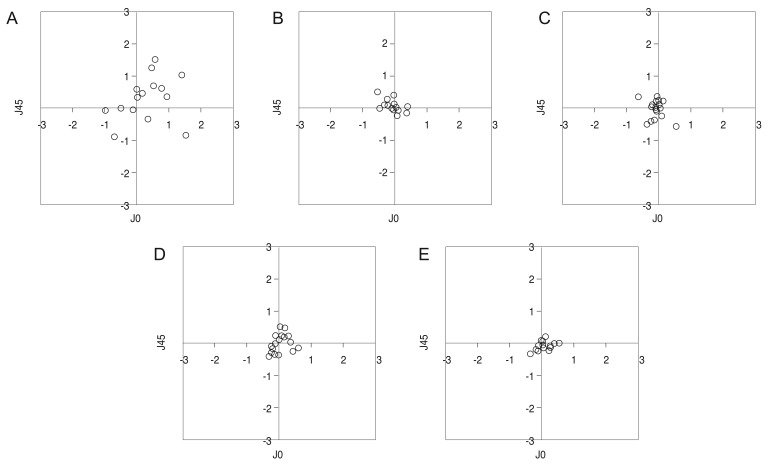
Vector components J0 and J45 decreased to nearly zero immediately after surgery, and they remained this low throughout the follow-up period (Table 3). Fig. 4A–4E shows how dispersed dots converged to zero 1 day after surgery, and this conversion was maintained until 3 months postsurgery.
IOL rotation
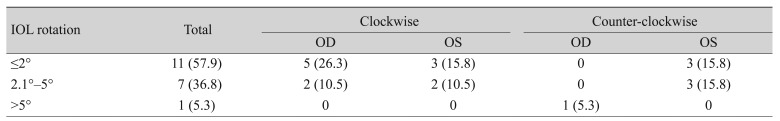
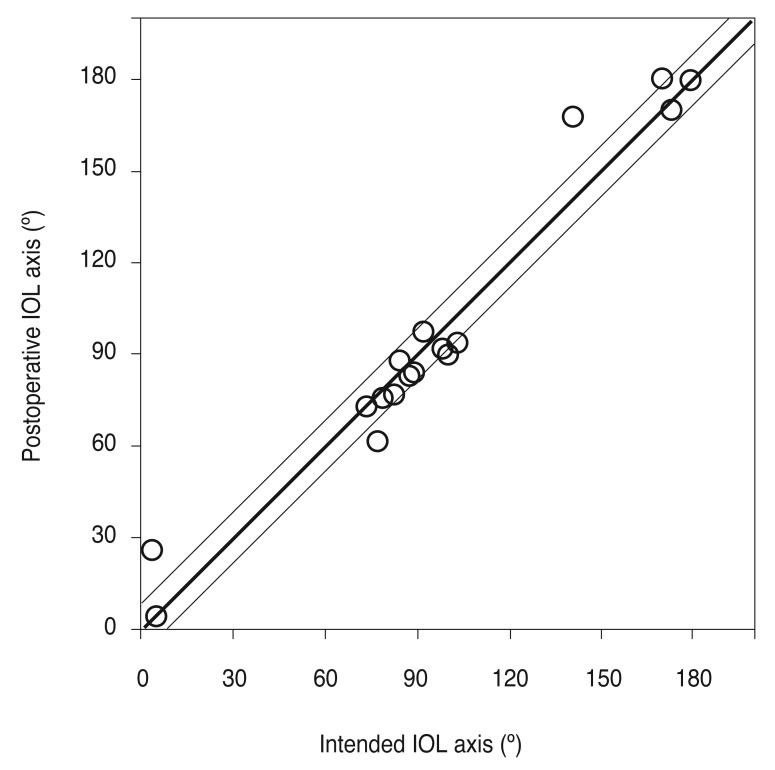
The mean absolute IOL rotation was 1.23° ± 1.27° (range, 0.06° to 4.55°) between 1 week and 1 month after surgery. Mean absolute IOL rotation was 1.81° ± 1.31° (range, 0.43° to 5.36°) between 1 week and 3 months after surgery, but these rotations were not statistically significant (p = 0.248 and p = 0.784, respectively). A rotation <2° was observed in 11 eyes (57.9%), and a rotation <5° was observed in 17 eyes (94.7%) (Table 4). One eye (5.3%) had an IOL rotation >5° (Table 4). Regarding the rotational direction, 36.8% were counter-clockwise, while 63.2% were clockwise (Table 4). The mean absolute misalignment compared with the intended IOL axis 1 week, 1 month, and 3 months after surgery was 4.65° ± 3.87°, 4.93° ± 3.28°, and 4.54° ± 3.18° (range, 0.02° to 10.23° 3 months postoperative) (Fig. 5).
Complications
No eyes had any intraoperative or postoperative complications that required a secondary intervention or secondary surgery to reposition the IOL. There were no sight-threatening complications over the 3-month follow-up period. No eyes had posterior capsule opacification (PCO) that required neodymium: YAG capsulotomy.
Discussion
Toric IOL has generally been used as a surgical option to correct corneal astigmatism during cataract surgery, and it has excellent visual and refractive effects. It can improve the quality of life of patients who have spectacle independence [610]. Among various toric IOLs, the AT Torbi 709M toric IOL has a bitoric optic design to correct high astigmatism and a 4-haptic plate design to minimize IOL rotation. In this study, the efficacy and stability of the AT Torbi 709M IOL in Asian patients with cataract and astigmatism were evaluated.
Our results revealed that postoperative SE was within ±0.50 D in 73.7% of patients and within ±1.00 D in 94.7% of patients. RAS was within ±0.50 D in 83.3% of patients and within ±1.00 D in all (100%) patients. Mean RAS 3 months after surgery was −0.54 ± 0.46 D (range, 0 to −1.75 D). These outcomes for Asian patients were similar to those previously reported after evaluating the same type of bitoric IOL in Caucasians. Kretz et al. [5] reported that 76% and 97% of eyes in German patients had postoperative SE within ±0.50 and ±1.00 D, respectively, with 86% of eyes having RAS of 0.50 D or less, while 95% of eyes had RAS of 1.00 D or less. Bascaran et al. [11] found that 92.7% and 75.6% of Spanish eyes have postoperative SE within ±1.00 D and ±0.50 D, respectively, with mean RAS after surgery of −0.43 ± 0.53 D. Asian eyes have been reported to have different ocular biometric parameters, including longer AL, shallower anterior chamber, and steeper cornea compared with Caucasian eyes [1213]. The Zeiss toric IOL online calculator appears to have been well optimized for accurately predicting IOL power for Asian eyes with shallow anterior chambers.
Zhu et al. [14] used the AcrySof toric IOL and found that UDVA (0.37 logMAR) and CDVA (0.08 logMAR) were significantly improved one year after surgery. Mean RAS values were within 0.50 D and 1.00 D in 38.7% and 84.0% of patients, respectively. Sheppard et al. [15] used the Tecnis toric IOL a nd reported a mean UDVA of 0.15 logMAR, with 95.4% of eyes having a mean SE within ±1.00 D of emmetropia. Their results indicate that visual and refractive outcomes from AT Torbi IOLs are comparable to or better than results of other toric IOLs. Such excellent outcomes suggest that bitoric IOLs can provide a high level of precision in refractive correction of corneal astigmatism after cataract surgery. The AcrySof IOL has a posterior toric optic, while the Tecnis IOL has an anterior toric optic. The AT Torbi IOL has 1.0 to 12.0 D cylindrical power with 0.5 steps and can precisely correct large amounts of astigmatism. The AcrySof toric IOL is available to correct astigmatism with cylindrical power of 1.50 to 6.00 D (in 0.75 steps) [1617]. The Tecnis toric IOL has 1.00, 1.50, 2.25, 3.00, and 4.00 D cylinder powers [15].
In our study, the mean absolute rotation of the IOL from 1 week to 3 months after surgery was 1.81° (range, 0.43° to 5.36°). Using the same toric IOL, a mean absolute IOL misalignment of 3.5° (range, 0° to 10°) was reported among German patients [5], and a mean toric IOL axis rotation of 4.42° (range, 0° to 16°) was reported among Spanish patients [11].
Rotational stability is primarily influenced by the interaction between IOL and capsular bag. Insufficient haptic size relative to the capsular bag was considered a key factor in early postoperative rotational instability [1819]. Kim et al. [7] measured the capsular bag diameter of Asian eyes and reported a mean capsular bag diameter of 11.30 mm on postoperative day 1, which was larger than that reported among Caucasian eyes (mean, 10.31 mm). Since capsular bag diameter in Asian eyes is larger in early postoperative days, greater rotational instability of the IOL might be expected. However, there appears to be no difference in rotational instability of the IOL between Asian and Caucasian eyes, possibly because the plate haptic IOL is 11.0 mm in length, which is long enough to be stable in the capsular bag of Asian eyes.
Sheppard et al. [15] used the Tecnis toric IOL and found that the mean absolute IOL misalignment at the 8-week follow-up visit was 3.4° (range, 0° to 12°). Zhu et al. [14] showed t hat t he m ean absolute r otation of t he AcrySof IOL one year after surgery was 8.83° ± 5.26° (range, 0.2° to 20.2°). Our rotation results are comparable to or better than those reported in most previous studies that used other toric IOLs. In comparison to open loop haptics, plate haptic IOLs are not as susceptible to compression effects from the capsular bag [19]. Lens epithelial cells can migrate through the positioning holes on plate haptic IOLs, thus anchoring the lens in place and improving its longterm stability [20]. Plate haptic IOLs have demonstrated excellent long-term stability [1921]. Given that the mean absolute misalignment (4.54°) after 3 months was not significantly different from that (1.81°) at 1 week, IOL misalignment might have occurred during IOL implantation, not postoperatively.
Our study has several limitations. First, we did not include a control group of patients who received other types of toric IOLs. Some errors may have been introduced into our comparisons with other studies as a consequence of different surgeons or different surgical procedures. Second, we did not evaluate the long-term outcome from PCO occurrence. Several studies have reported that PCO occurrence was significantly lower with hydrophobic acrylic IOLs compared with hydrophilic acrylic IOLs [22]. Although t he AT Torbi t oric IOL is m ade of hydrophilic acrylic material, it has a hydrophobic surface, which may offer an important advantage against PCO. Long-term evaluation is needed to determine PCO occurrence in future studies. Third, we expected to find differences in clinical outcomes from the AT Torbi toric IOL between Caucasian and Asian patients, but we did not find significant differences. However, because of the ethnic differences in capsular bag diameter and ocular biometric parameters, it is doubtful that the 4-haptic IOL could be well placed in the capsular bag, and the Zeiss toric IOL online calculator predicts IOL power accurately in Asians. Thus, we believe it would be worthwhile to evaluate the clinical outcomes from 4-haptic bitoric IOL for Asian patients.
The AT Torbi 709M bitoric IOL is effective for improving distance vision and for reliably correcting corneal astigmatism in Asian eyes with cataract and corneal astigmatism.
Acknowledgements
This study was supported by a grant from Carl Zeiss Meditec Inc. (project no. 06-2015-1900).
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.