Development of Submacular Hemorrhage in Neovascular Age-related Macular Degeneration: Influence on Visual Prognosis in a Clinical Setting
Article information
Abstract
Purpose
This study aimed to evaluate changes in visual acuity before and after the development of submacular hemorrhage secondary to neovascular age-related macular degeneration (AMD) and to compare the visual outcomes between patients with and without hemorrhage.
Methods
This retrospective observational study included 124 patients with neovascular AMD. Patients who developed a submacular hemorrhage involving the fovea were included in the hemorrhage group (n = 55). Patients with no sign of submacular hemorrhage during the follow-up period were included in the no-hemorrhage group (n = 69). Visual outcomes were compared between the two groups.
Results
The logarithm of the minimal angle of resolution best-corrected visual acuity (BCVA) before the development of submacular hemorrhage, once the hemorrhage had developed, and 6 months after the development of hemorrhage was 0.59 ± 0.45, 1.24 ± 0.57, and 0.99 ± 0.64, respectively. BCVA was significantly worse 6 months after the hemorrhage compared to before the hemorrhage (p < 0.001). The BCVA before the development of hemorrhage (measured at a mean of 12.9 months after diagnosis) was comparable to that of the no-hemorrhage group (mean, 0.58 ± 0.37 at a mean of 12.4 months). However, the BCVA 6 months after identification of hemorrhage (mean, 21.5 months) was significantly worse in the hemorrhage group than in the no-hemorrhage group (mean, 0.73 ± 0.44 at mean 21.2 months) (p = 0.018).
Conclusions
Visual acuity was significantly worse after hemorrhage than before hemorrhage, even after treatment. In addition, patients with submacular hemorrhage had markedly worse visual outcomes than patients without hemorrhage. This result suggests that the development of hemorrhage during the treatment course of neovascular AMD has a devastating effect on visual prognosis.
The prognosis of submacular hemorrhage secondary to neovascular age-related macular degeneration (AMD) is generally poor [123]. However, previous studies have shown that severe deterioration in visual function can be partially prevented by administering various treatment modalities [4567891011121314]. Anti-vascular endothelial growth factor (VEGF) monotherapy is an effective treatment for submacular hemorrhage secondary to neovascular AMD [7891011121314]. However, most previous studies included patients who presented with submacular hemorrhage. Thus, the visual acuity at the time of hemorrhage was compared to the visual acuity after treatment. In submacular hemorrhage cases, resolution of the hemorrhage itself may lead to an improvement in visual acuity, regardless of whether the underlying retinal function was restored. Thus, to more accurately evaluate the fundamental influence of submacular hemorrhage on retinal function, visual acuity after treatment should be compared with visual acuity before the development of hemorrhage. In addition, the visual changes should be compared with an appropriate control group.
The purpose of the present study was to evaluate the influence of submacular hemorrhage on visual prognosis in neovascular AMD. The visual outcomes of these patients were compared with those of patients diagnosed with neovascular AMD who did not develop submacular hemorrhage during the follow-up period.
Materials and Methods
This retrospective, observational case series adhered to the tenets of the Declaration of Helsinki. The study was approved by the institutional review board of Kim's Eye Hospital, Seoul and Konyang University Hospital, Daejeon, South Korea (A-2013-023). The informed consent was not obtained in this retrospective study.
We conducted a computerized search for patients who were newly diagnosed with neovascular AMD between September 2009 and December 2012 at our institution. Patients who developed submacular hemorrhage involving the fovea in at least one-disc area during the follow-up period were included. Other inclusion criteria were 1) anti-VEGF monotherapy after development of hemorrhage and 2) a 6-month or longer follow-up period after the development of hemorrhage.
To compare the visual acuity outcomes between patients with and without submacular hemorrhage, patients who developed submacular hemorrhage were categorized into the hemorrhage group. The no-hemorrhage group included patients who had completed at least 20 months of follow-up, did not have submacular hemorrhage during the follow-up, had no history of intraocular surgery including cataract surgery between 11 and 24 months after diagnosis, and who received anti-VEGF monotherapy between 11 and 24 months after diagnosis. Patients who had undergone photodynamic therapy (PDT) before 11 months after diagnosis were not excluded. Since the development of hemorrhage is considered a re-activation of the lesion, patients with at least one episode of recurrence of fluid between 11 and 24 months after the initial diagnosis were included.
Each patient had undergone a comprehensive ophthalmologic examination, including measurements of best-corrected visual acuity (BCVA), 90-diopter lens slit-lamp biomicroscopy, fundus photography, fluorescein angiography, and spectral-domain optical coherence tomography (OCT; Spectral OCT/SLO, OTI Ophthalmic Technologies, Miami, FL, USA). Indocyanine green angiography (ICGA) with a confocal laser-scanning system (HRA-2; Heidelberg Engineering, Dossenheim, Germany) was performed at the discretion of each physician. The exclusion criteria included severe media opacity, history of intraocular surgery except cataract surgery, evidence of a macroaneurysm, proliferative diabetic retinopathy, or retinal vascular occlusion. These exclusion criteria were also applied to the no-hemorrhage group.
The main outcome measure was BCVA. BCVA was measured at the initial diagnosis of neovascular AMD, before the development of submacular hemorrhage, once the hemorrhage had developed, and 6 months after the development of hemorrhage. BCVA before the development of hemorrhage was defined as the value measured at the most recent hospital visit before the development of submacular hemorrhage. If the hemorrhage had developed just after the initial diagnosis, the BCVA at diagnosis was recorded as the value before the development of hemorrhage. Visual acuities were converted to the logarithm of the minimal angle of resolution (logMAR) values for analysis.
The extent of hemorrhage and central foveal thickness (CFT) were estimated by analyzing fundus photographs and OCT images, respectively. CFT was defined as the distance between the internal limiting membrane and Bruch's membrane at the fovea and was measured manually using virtual c alipers provided as part of a n O CT software program. The combined thickness of the subretinal and sub-retinal pigment epithelial hemorrhage was defined as the thickness of the hemorrhage and was measured at the fovea as the distance between the outermost point of the neurosensory retina and Bruch's membrane. The baseline extent of hemorrhage and CFT were defined as the values measured at the time when the hemorrhage had developed. In this study, we considered the possibility that measurements of hemorrhages exceeding 20-disc areas and CFT exceeding 1,500 µm could be inaccurate. Therefore, 20-disc areas of hemorrhage and 1,500 µm of CFT were considered as threshold values; lesions exceeding this range were considered as 20-disc areas in size or 1,500 µm in depth. The extent of hemorrhage, CFT, and the thickness of hemorrhage at the fovea were estimated by two independent examiners. The mean values of the measurements obtained by the two examiners were used for analysis.
The results of ICGA were also analyzed by two independent examiners. Patients with neovascular AMD were classified as having either typical neovascular AMD or polypoidal choroidal vasculopathy (PCV) on the basis of ICGA findings. Patients exhibiting polypoidal lesions with or without branching vascular networks were diagnosed with PCV. The other patients were classified as having typical neovascular AMD. The unclassified group included patients without an ICGA result or poor ICGA image quality that may preclude accurate diagnosis of underlying lesion. Any disagreement was settled by discussion between the two examiners. The following analyses were performed.
Changes in BCVA and factors associated with visual prognosis in the hemorrhage group
We compared BCVAs measured before the development of submacular hemorrhage, once the hemorrhage had developed, and 6 months after the development of hemorrhage. In addition, patients were divided into two groups according to the degree of visual deterioration 6 months after hemorrhage; patients who experienced ≥3 lines of deterioration in BCVA compared to the value measured before hemorrhage were included in the worse visual outcomes group. The remaining patients were included in the better visual outcomes group. The following variables were compared between the two groups: BCVA before development of hemorrhage, baseline extent of hemorrhage, thickness of hemorrhage, period between diagnosis and development of submacular hemorrhage, and number of anti-VEGF injections.
Comparison between the hemorrhage group and the no-hemorrhage group
Age, sex, and the proportion of patients in each diagnosis group (typical neovascular AMD vs. PCV vs. unclassified) were compared between the hemorrhage group and the no-hemorrhage group. The time points used to compare visual acuity outcomes were defined as follows. In the hemorrhage group, the first time point was before the development of submacular hemorrhage, and the second time point was 6 months after the development of hemorrhage. The first and second time points in the hemorrhage group were measured as 12.9 ± 8.1 and 21.5 ± 8.9 months, respectively, after initial diagnosis. For appropriate comparison, the first and second time points of the no-hemorrhage group were defined as hospital visits that were closest to 13 and 22 months after diagnosis. The period between diagnosis and each corresponding time point was compared between the two groups. BCVA at each corresponding time point and number of anti-VEGF injections administered between the first and second time points were compared between groups as well.
Statistics
Statistical analyses were performed using a commercially available software package (SPSS ver. 12.0; SPSS Inc., Chicago, IL, USA). Differences in BCVA at various time points were analyzed using a repeated-measures analysis of variance, and individual comparisons were performed using Bonferroni's method. Differences between groups were analyzed using the chi-square test or an independent-samples t-test with or without Bonferroni's correction. A p-value <0.05 was considered significant.
Results
A total of 124 patients (124 eyes) met the eligibility criteria. Fifty-five patients were included in the hemorrhage group and 69 patients were included in the no-hemorrhage group (Table 1). In the hemorrhage group, the mean age was 67.9 ± 7.8 years. Nineteen (34.5%) and 24 (43.6%) patients from the hemorrhage group were ultimately diagnosed with typical neovascular AMD and PCV, respectively. The remaining 12 patients (21.8%) were included in the unclassified group. The mean period between diagnosis and development of submacular hemorrhage was 15.5 ± 8.9 months. Fig. 1 shows a representative case of a patient who experienced submacular hemorrhage.
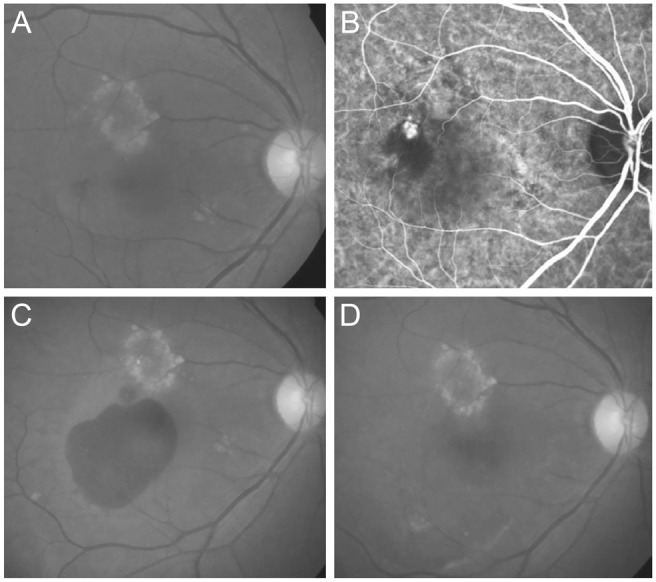
Images taken (A,B) at diagnosis, (C) once the hemorrhage had developed, and (D) 6 months after the development of hemorrhage in patient diagnosed with polypoidal choroidal vasculopathy. The best-corrected visual acuity measured at each time point was 20 / 100, 20 / 200, and 20 / 200, respectively. (A,C,D) Fundus photography and (B) indocyanine green angiography.
Before the development of submacular hemorrhage, all 55 patients were treated with anti-VEGF, and 7 of them also received PDT. After development of hemorrhage, 26 patients (47.3%) were treated with ranibizumab, 22 (40.0%) were treated with bevacizumab, and the remaining 7 (12.7%) were treated with both agents. The mean CFT was 612.5 ± 316.8 µm when hemorrhage developed.
Changes in BCVA and factors associated with visual prognosis in the hemorrhage group
In the hemorrhage group, the mean BCVA at initial diagnosis, before development of hemorrhage (the first time point), once hemorrhage had developed, and 6 months after development of hemorrhage (the second time point) was 0.56 ± 0.42 (Snellen equivalent, 20 / 72), 0.59 ± 0.45 (20 / 77), 1.24 ± 0.57 (20 / 347), and 0.99 ± 0.64 (20 / 195), respectively (Fig. 2). BCVA differed significantly between the three time points (p < 0.001). BCVA was significantly better 6 months after the hemorrhage than at the time of hemorrhage (p = 0.001). However, BCVA was still significantly worse at 6 months after the hemorrhage than before the hemorrhage (p < 0.001).
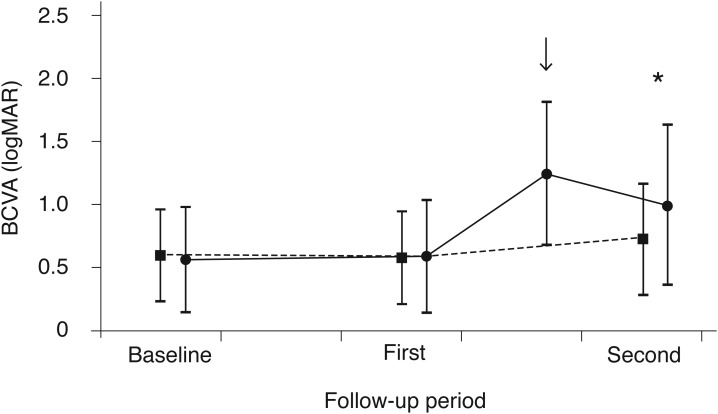
Changes in the logarithm of the minimal angle of resolution best-corrected visual acuity (BCVA) in eyes with (hemorrhage group; solid line, closed circles, n = 55) and without submacular hemorrhage (no-hemorrhage group; dashed line, closed squares, n = 69), according to follow-up. In the hemorrhage group, the mean BCVA at initial diagnosis, at the first time point, after development of hemorrhage (arrow), and at the second time point was 0.56 ± 0.42, 0.59 ± 0.45, 1.24 ± 0.57, and 0.99 ± 0.64, respectively. In the no-hemorrhage group, the mean BCVA at initial diagnosis, at the first time point, and at the second time point was 0.60 ± 0.37, 0.58 ± 0.37, and 0.73 ± 0.44, respectively. The BCVA at the first time point was comparable between the two groups (p = 1.000, independent-samples t-test with Bonferroni's correction). However, the BCVA at the second time point was significantly worse in the hemorrhage group than in the no-hemorrhage group (*p = 0.018, independent-samples t-test with Bonferroni's correction). Each time point was defined as follows: baseline, once neovascular age-related macular degeneration was diagnosed; first, the first time point; and second, the second time point. logMAR = logarithm of the minimal angle of resolution.
Compared to BCVA measured before hemorrhage, an improvement in visual acuity or restoration of visual acuity to the same level was achieved in only 14 patients (25.5%). BCVA deteriorated by <3 lines in 17 patients (30.9%) and by ≥3 lines in the remaining 24 (43.6%). As a result, the better visual outcome group included 31 patients, whereas the worse visual outcome group included 24 patients. There was no significant difference in the variables between the two groups (Table 2).
Comparison between the hemorrhage group and the no-hemorrhage group
In the no-hemorrhage group, the mean age was 70.8 ± 8.2 years. Thirty (43.5%) and 29 (42.0%) patients were ultimately diagnosed with typical neovascular AMD and PCV, respectively. The remaining 10 patients (14.5%) were included in the unclassified group. The mean first and second time points in the control group were 12.4 ± 0.9 months and 21.2 ± 1.4 months, respectively. Before the first time point, all 69 patients were treated with anti-VEGF, and 6 of them also had a history of PDT. Between the first and the second time points, 28 patients (40.6%) were treated with ranibizumab, 31 (44.9%) were treated with bevacizumab, and the remaining 10 (14.5%) were treated with both agents. The mean number of anti-VEGF injections administered between the first and the second time points was 2.7 ± 0.9 and 2.6 ± 1.1 in the hemorrhage group and in the no-hemorrhage group, respectively. The number of anti-VEGF injections did not differ between the groups (p = 0.294). Results of comparisons between the hemorrhage group and the no-hemorrhage group are summarized in Table 1. The no-hemorrhage group was significantly older than the hemorrhage group (p = 0.046). Other features were not different between the two groups.
Fig. 2 compares the changes in BCVA between the hemorrhage and the no-hemorrhage groups. In the no-hemorrhage group, the mean BCVA at initial diagnosis, at a mean duration of 12.4 ± 0.9 months (the first time point), and at a mean duration of 21.2 ± 1.4 months (the second time point) was 0.60 ± 0.37 (Snellen equivalent, 20 / 79), 0.58 ± 0.37 (20 / 76), and 0.73 ± 0.44 (20 / 107), respectively. Although BCVA measured at the first time point was not different between the groups (p = 1.000), BCVA at the second time point was significantly worse in the hemorrhage group than in the control group (p = 0.018).
Discussion
In the present study, visual acuity improved significantly after the development of hemorrhage. However, visual acuity 6 months after hemorrhage was significantly worse than that before hemorrhage. More than 40% of patients lost ≥3 lines of visual acuity despite treatment. As a result, the visual outcomes of patients who had submacular hemorrhage were significantly worse than those of patients who did not.
In this study, the outcomes of the patients were compared with those of an appropriate control group, i.e., patients diagnosed with neovascular AMD but without submacular hemorrhage. Because the development of submacular hemorrhage may represent lesion recurrence, only patients who had received anti-VEGF injections between 11 and 24 months after diagnosis were included in the no-hemorrhage group. The time points of 11 and 24 months were defined by the authors based on the first (mean, 12.9 months) and second (mean, 21.5 months) time points in the hemorrhage group, with a slight extension of the period from 12.9 to 21.5 months to 11 to 24 months.
In the present study, the second time point was set at 6 months after the development of hemorrhage. This was determined based on previous studies [81012], as the extent of hemorrhage and CFT markedly decrease during the first several months [81012]. In a study by Kim et al. [8], the mean extent of hemorrhage was 6.4 disc areas at diagnosis a nd 0.3 d isc a reas a t 6 months. In a ddition, C FT markedly decreased during the first 6 months, but remained relatively stable between 6 and 12 months. In a study by Stifter et al. [10], the mean extent of hemorrhage was 19.7 mm2 at diagnosis and 2.5 mm2 at 16 weeks. In addition, mean foveal thickness did not markedly change from 12 weeks (mean, 223 µm) to 16 weeks (mean, 229 µm). These results suggest that most hemorrhages resolve during the first 6 months; therefore, the second time point was set 6 months after the development of hemorrhage.
Visual acuity measured at the first time point was comparable between the hemorrhage group and the no-hemorrhage group. However, BCVA 6 months after the development of hemorrhage (mean duration of 21.5 months after initial diagnosis) was significantly worse than that measured at a mean duration of 21.2 months in the control group, despite a comparable number of anti-VEGF injections administered between the first and second time points. This result suggests the negative influence of development of submacular hemorrhage on visual prognosis in neovascular AMD.
Cho et al. [15] previously reported the incidence and risk factors of massive submacular hemorrhage (>4 disc diameter in size) in PCV. In that study, the visual prognosis was significantly worse in patients with massive submacular hemorrhage (mean, 1.34 ± 0.66 at final visit) than in patients without it (mean, 0.63 ± 0.53 at final visit). When comparing visual acuity between patients with and without submacular hemorrhage, we believe that the present study has several advantages over the study of Cho et al. [15]. It is well-known that recurrence of fluid after initial treatment does not occur in some proportion of patients with neovascular AMD, and the visual prognosis is generally good in such cases [1617]. To avoid the potential influence of the absence of recurrence, only patients who experienced recurrence between the first and second time points were included in the no-hemorrhage group. In addition, all our patients were treated with anti-VEGF monotherapy during the study period, whereas various treatment modalities were used in the study of Cho et al. [15]. Nevertheless, we believe that the similar results in the previous study [15] and the present study strengthen our conclusion.
Anti-VEGF monotherapy is effective in treating submacular hemorrhage secondary to neovascular AMD [7891011121314]. Moreover, one recent study demonstrated that the efficacy of additional pneumatic displacement is limited when anti-VEGF therapy is performed for submacular hemorrhage [18]. Therefore, the difference in visual outcomes between patients with and without hemorrhage may be mainly attributed to the nature of submacular hemorrhage itself rather than the limited efficacy of anti-VEGF therapy in this condition.
The definite negative influence of submacular hemorrhage on the visual prognosis of neovascular AMD begs two important questions. The first question is whether it is possible to identify high-risk patients or to predict the development of submacular hemorrhage. The second more fundamental and practical question is whether we can prevent the development of submacular hemorrhage during the course of treatment for neovascular AMD. Unfortunately, the answers to these questions are beyond the scope of the present retrospective study. Further studies with more controlled designs will be necessary to address these issues.
The strength of the present study is that visual changes before and after submacular hemorrhage were compared with those of an appropriate control group. However, this study has obvious limitations. In this retrospective study in a clinical setting, a strict follow-up and treatment protocol was not used, and the number of anti-VEGF injections was not controlled. In addition, two different anti-VEGF agents were used at the discretion of the treating physician. This may have influenced the study results. All patients were treated with anti-VEGF monotherapy after the development of hemorrhage. Thus, the results of the present study may not be valid for patients receiving other treatment, such as pneumatic displacement or photodynamic therapy. Lastly, patients with at least one episode of fluid recurrence between 11 and 24 months after the initial diagnosis were included in the no-hemorrhage group. Although the first and the second time points were comparable between the hemorrhage and no-hemorrhage groups, the 11- and 24-month time points were arbitrarily defined by the authors. In addition, the first and the second time points were also arbitrarily defined by the authors. Although the results of previous studies suggest that most hemorrhages resolve during the first 6 months, the influence of residual hemorrhage on the study results may not be completely accounted for [81012].
In summary, once submacular hemorrhage developed during the course of treatment for neovascular AMD, visual acuity was not completely restored in the majority of patients. Therefore, the visual prognosis of patients who had submacular hemorrhage was discouraging compared with that of patients who had no hemorrhage. Further efforts to establish the optimal approach to prevent hemorrhage as well as effective treatment modalities would be of great value.
Acknowledgements
This study was supported by Kim's Eye Hospital Research Center and Aju Pharmaceutical.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.