A Comparative Study of Topical Mitomycin C, Cyclosporine, and Bevacizumab after Primary Pterygium Surgery
Article information
Abstract
Purpose
To compare the recurrence rates and complications associated with instillation of topical mitomycin C, cyclosporine, and bevacizumab after primary pterygium surgery.
Methods
Between July 2013 and June 2014, we performed surgery using the bare sclera method on 132 eyes (132 patients) with primary pterygium. We randomly selected 33 eyes (33 patients) and treated them with artificial tears four times a day for three months, 29 eyes (29 patients) were treated with topical 0.02% mitomycin C four times a day for five days, 34 eyes (34 patients) were treated with topical 0.05% cyclosporine four times a day for three months, and 36 eyes (36 patients) were treated with topical 2.5% bevacizumab four times a day for three months after surgery. We prospectively determined the recurrence rates of pterygium and complications at the six-month follow-up examination.
Results
At six months after surgery, the recurrence rates in each group were as follows: 45.5% (15 eyes) in the control group, 10.3% (three eyes) in the mitomycin C group, 20.6% (seven eyes) in the cyclosporine group, and 41.7% (15 eyes) in the bevacizumab group (p = 0.004). No serious complications, except subconjunctival hemorrhages, were observed in any group.
Conclusions
Groups receiving topical 0.02% mitomycin C and 0.05% cyclosporine after surgery showed lower recurrence rates than the control group; however, no difference in recurrence rate was observed between the control group and the group receiving topical 2.5% bevacizumab after surgery.
Pterygium is a disease associated with proliferation of the fibrovascular tissues of the conjunctiva into the cornea and is related to factors such as ultraviolet light exposure, chronic stimulation, inflammation, climate, and genetics [1]. The first line of treatment for primary pterygium is surgical excision, and despite postoperative adjuvant therapy using mitomycin C, cyclosporine, β-irradiation, argon laser, and bevacizumab, recurrence rates remain high [23456].
Mitomycin C is a metabolic inhibitor extracted from Streptomyces caespitosus that inhibits DNA synthesis [7]. Cyclosporine is an immunosuppressant that selectively suppresses T-helper cells, controls interleukin synthesis and secretion, and inhibits vascular endothelial growth factor (VEGF) [8]. Bevacizumab is an anti-VEGF antibody that inhibits angiogenesis. Each agent has been studied as an adjuvant therapy to inhibit post-surgery pterygium recurrence [9].
Mitomycin C is generally used as an adjuvant therapy after surgery, but its use is limited because of severe side effects such as scleral necrosis, corneal perforation, corneal edema, secondary glaucoma, corneal calcification, and cataracts [1011]. Topical cyclosporine and bevacizumab are relatively effective in inhibiting recurrence, but topical cyclosporine causes minor complications such as irritation, hyperemia and rarely, scleromalacia [12]. Bevacizumab can cause severe systemic problems such as endophthalmitis and arterial thromboembolic events [1314]. Thus, further studies are needed to evaluate the efficacy and safety of these agents [89].
In this study, we instilled mitomycin C, cyclosporine, or bevacizumab after surgical excision of primary pterygium and compared the recurrence rates and complications among the therapies in order to determine the most effective postoperative adjuvant therapy.
Materials and Methods
This prospective, randomized, single-center study was performed in accordance with the Helsinki Declaration of 1975 and its 1983 revision and was approved by the review board at the Veterans Health Service Medical Center in Seoul, Korea. All patients provided informed consent after receiving a full explanation of the treatment process, risks involved, and available alternatives.
Between July 2013 and June 2014, 132 patients (132 eyes) whose condition was diagnosed as primary pterygium underwent surgery using the bare sclera method at the center and were followed up for six months after surgery. We excluded patients with uncontrollable systemic diseases such as hypertension, diabetes, or cardiovascular diseases; diseases of the eye surface such as conjunctivitis and keratitis; a history of eye surgery within the previous six months; or hypersensitivity reaction to one of the eye drops.
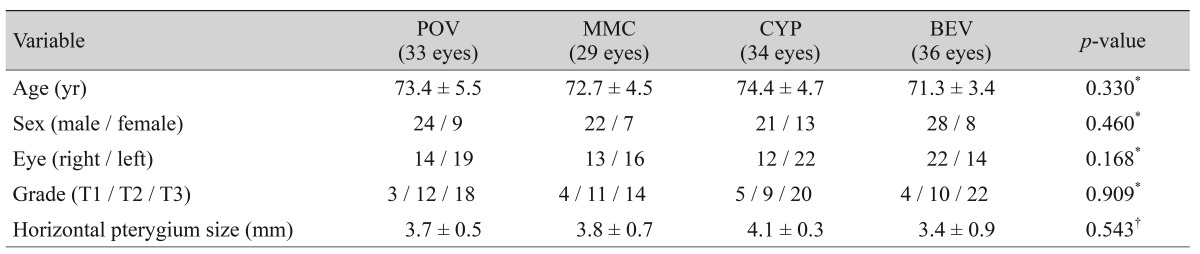
We recorded each patient's medical history and visual acuity, measured the intraocular pressure, and performed an anterior segment slit lamp examination and measurements of corneal endothelial cell density before surgery. Pterygium was classified before surgery according to the classification proposed by Tan et al. [15]. Pterygium was classified as T1 (atrophic) when the episcleral blood vessels could be clearly distinguished below the pterygium body, as T2 (intermediate) when the episcleral blood vessels were partially visible below the pterygium body, and as T3 (fleshy) when the episcleral blood vessels were completely hidden from sight by the pterygium body. In addition, we measured the distance from the corneal limbus to the head of the pterygium in each patient.
A single surgeon performed excision of the pterygium using the bare sclera method. Antibiotic ointment (0.03% tobramycin; Toravin, Han Lim Pharm, Seoul, Korea) was instilled immediately after surgery, and a pressure patch was applied for one day. After removal of the pressure patch, all patients were administered antibiotic eye drops (0.3% gatifloxacin; Gatiflo, Handok, Seoul, Korea) and steroid eye drops (0.1% fluorometholone; Fumelon, Han Lim Pharm) four times a day for the first week. Only the steroid eye drops were used twice a day for the next three weeks. Subsequently, patients were randomly separated into four adjuvant therapy groups, as follows: artificial eye drops (Povidon, Han Lim Pharm) four times a day for three months after surgery (control group), 0.02% topical mitomycin C (Mitomycin-C Kyowa; Kyowa Hakko Kirin, Seoul, Korea) four times a day for five days after surgery, topical 0.05% cyclosporine (Restasis; Allergan, Irvine, CA, USA) four times a day for three months after surgery, or topical 2.5% bevacizumab (Avastin; Genentech, South San Francisco, CA, USA) instilled four times a day for three months after surgery. Patients in the bevacizumab group were specifically instructed to change eye drops once a month and to store the eye drops at a cold temperature (2℃ to 8℃).
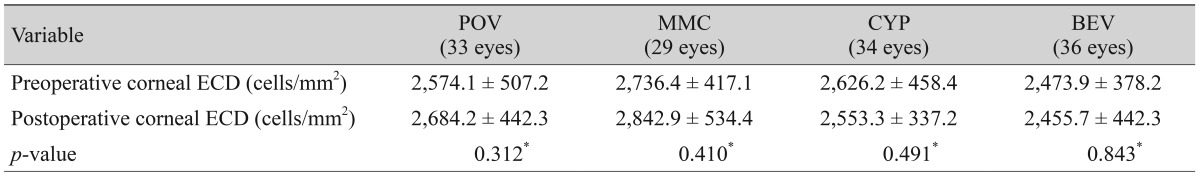
To achieve double blinding, an observer other than the operating surgeon monitored the progress at one day, one week, one month, three months, and six months after surgery. Recurrence after surgery was determined using the G0-G3 classification proposed by Prabhasawat et al. [16]. According to the anterior segment slit lamp examination, grade 0 was defined as no recurrence; grade 1 was defined as thin episcleral blood vessels, not accompanied by fibrosis, observed around the excised area; grade 2 was defined as fibrovascular proliferation limited to the sclera in the excised area; grade 3 was defined as fibrovascular proliferation crossing the corneal limbus. Grades 2 and 3 were defined as recurrence. Additionally, we investigated patients in whom complications occurred during the follow-up period, measured the corneal endothelial cell density at six months after surgery, and compared these values with those observed before the surgery.
All statistical analyses were carried out using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). A p-value less than 0.05 was regarded as statistically significant. Chi-square test and one-way analysis of variance (ANOVA) were used to compare demographic variables. To evaluate the possible corneal endothelial toxicity and stability of each group, paired t-tests were used to compare preoperative and postoperative corneal endothelial cell densities in each group. One-way ANOVA was used to assess the difference in recurrence rates at three and six months of follow-up. If statistical significance was indicated, a post hoc comparison was performed using the least significant difference (LSD) test in each group.
Results
We included 132 patients (132 eyes), of whom 95 (72.0%) were men and 37 (28.0%) were women; their average age was 73.9 ± 6.7 years (range, 64 to 86 years). A total of 33 patients (33 eyes) received artificial eye drops, 29 patients (29 eyes) received mitomycin C, 34 patients (34 eyes) received cyclosporine, and 36 patients (36 eyes) received bevacizumab. No statistically significant differences were observed in age, gender, laterality, preoperative pterygium grade, or horizontal distance from the corneal limbus to the pterygium head among the groups (Table 1).
During the follow-up period, subconjunctival hemorrhage occurred in two eyes in the control group, one eye in the mitomycin C group, one eye in the cyclosporine group, and two eyes in the bevacizumab group. All patients completely recovered, and no other abnormal ocular or systemic complications were observed during the six-month follow-up period. There was no significant difference in preoperative and postoperative corneal endothelial cell densities (Table 2).
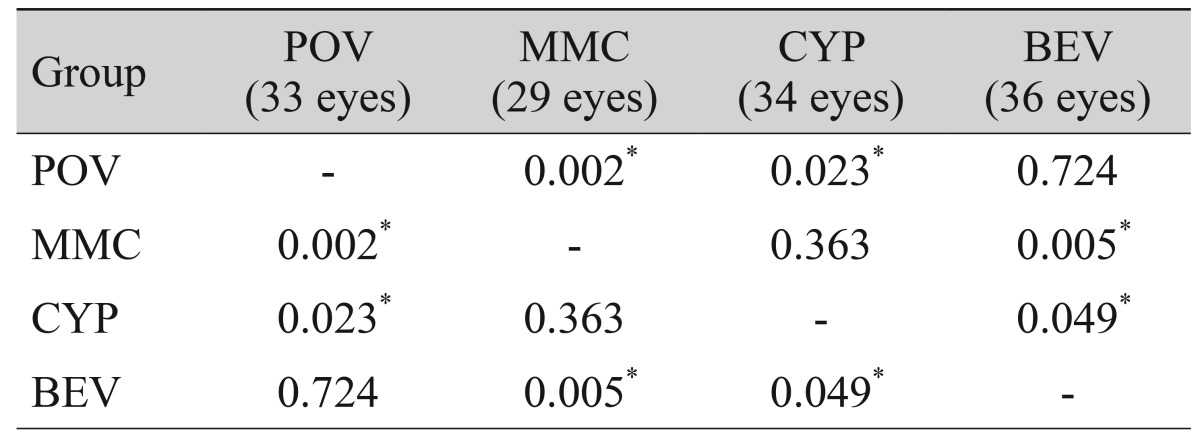
No recurrence was observed at one d ay, one week, or one month after surgery. However, at three months, recurrence was observed in eight eyes (24.2%) in the control group, one eye (3.4%) in the mitomycin C group, three eyes (8.8%) in the cyclosporine group, and two eyes (5.6%) in the bevacizumab group. Because recurrence rates differed significantly at three months (p = 0.027), we performed post hoc analysis using the LSD test. All treatment groups showed significantly lower recurrence rates compared to the control group but did not differ from each other (Table 3). At six months, recurrence was observed in 15 eyes (45.5%) in the control group, three eyes (10.3%) in the mitomycin C group, seven eyes (20.6%) in the cyclosporine group, and 15 eyes (41.7%) in the bevacizumab group. Because the recurrence rate at six months differed significantly in all groups (p = 0.004), we performed post-hoc analysis using the LSD test and found that the mitomycin C and cyclosporine groups had significantly lower recurrence rates compared to the control and bevacizumab groups. Recurrence rate did not differ between the control and bevacizumab groups (Table 4).
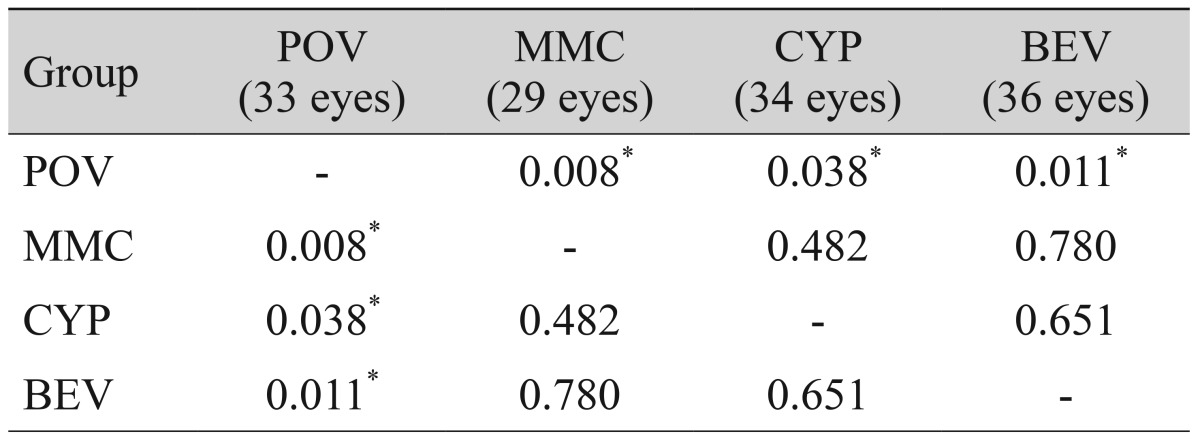
Post-hoc (LSD) comparisons of recurrence rates according to adjuvant treatment at three months (p-value)
Discussion
Recurrence rates after surgical treatment for pterygium can show large differences depending on preoperative treatment, method, surgeon experience, and postoperative adjuvant therapy. Although previously reported rates of recurrence vary, the overall recurrence rate is relatively high at 19.4% to 75.0% [1]. Various attempts have been made to lower this rate after pterygium surgery because recurrence is associated with complications such as cosmetic problems, inflammatory changes, and oculomotor disorders or diplopia resulting from adhesion [23456].
Mitomycin C is an antibiotic and anti-carcinogenic compound extracted from Streptomyces caespitosus that suppresses the proliferation of fibroblasts by inhibiting DNA synthesis [7]. Kunitomo and Mori first used mitomycin C in 1962; since then, it has been widely employed to prevent recurrence after pterygium excision surgery [10]. Mitomycin C also acts on normal tissues; however, complications such as scleral necrosis, corneal perforation, corneal edema, secondary glaucoma, corneal calcification and cataracts are possible, and it thus must be used with caution [11].
Pterygium is recognized as a problem related to cell growth rather than cellular degeneration [17], which has led to studies of whether cyclosporine is effective in suppressing pterygium tissue and preventing recurrence. Cases of cyclosporine use after pterygium excision have usually involved minor complications such as irritation, burning sensation, hyperemia, and a small percentage of scleromalacia [12]. Turan-Vural et al. [3] divided 36 eyes (34 patients) with primary pterygium into two groups and reported that the rate of recurrence was 44.4% in the group that received pterygium surgery using the bare sclera method alone but was 22.2% in the group receiving postoperative instillation of 0.05% cyclosporine. The cyclosporine-treated group showed no adverse reaction other than a mild burning sensation and irritation upon application. For 31 patients (62 eyes) diagnosed with bilateral pterygium, Yalcin Tok et al. [18] instilled 0.05% cyclosporine in the right eye, using the left eye as a control, a nd reported a rate of recurrence of 12.9% in the right eye and 45.2% in the left eye, which indicated that cyclosporine was effective in reducing pterygium recurrence.
Bevacizumab, a recombinant human monoclonal antibody, is an inhibitor that binds to all biologically active forms of VEGF. In 2004, the US Food and Drug Administration approved the drug for the treatment of metastatic colorectal cancer. In addition, bevacizumab showed promising results in ophthalmology for the treatment of many diseases related to angiogenesis, such as macular degeneration related choroidal neovascularization, proliferative diabetic retinopathy, and retinal vein occlusion [1920]. However, several systemic complications such as gastrointestinal perforation, hypertension, arterial hemorrhage, impaired wound healing, bleeding, endophthalmitis, and arterial thromboembolic events have been reported following subconjunctival or intravitreal injections [1314]. Assuming that VEGF is involved in proliferation and angiogenesis of f ibrovascular tissues, which causes the recurrence of pterygium, use of bevacizumab could reduce the recurrence rate. Motarjemizadeh et al. [21] enrolled 90 patients (90 eyes) who underwent pterygium excision and categorized them into three groups. At 24 hours after surgery, group II and group III received a 5 and 10 mg/mL dose of topical bevacizumab, respectively, whereas patients in group I were administered only a placebo starting one day after surgery. Participants were instructed to instill their topical medicines four times a day for one week. These authors concluded that 10 mg/mL of topical bevacizumab was more efficacious than 5 mg/mL in preventing pterygium recurrence. Ozgurhan et al. [9] divided 44 patients (44 eyes) who underwent excision of recurrent pterygium with conjunctival autograft into two groups, one of which was instilled with bevacizumab four times a day for a month, starting one month after surgery. Bevacizumab failed to reduce recurrence but effectively inhibited angiogenesis. Suh and Choi [22] divided 54 patients (54 eyes) who underwent primary pterygium surgical excision into two groups and, after administering subconjunctival bevacizumab injections to one group, reported that it failed to affect recurrence but successfully inhibited proliferation of fibrovascular tissues.
In this study, we investigated whether topical mitomycin C, cyclosporine, or bevacizumab is more effective among adjuvant instillation therapies in reducing recurrence rates after pterygium surgery, compared to a control group using artificial tears. To the best of our knowledge, this study is the first to compare these three treatments after primary pterygium surgery. Of these adjuvants, topical mitomycin C is most commonly used, although cyclosporine has recently received increasing attention. We used the G0-G3 classification proposed by Prabhasawat et al. [16] and measured the extent of pterygium proliferation and recurrence rates for each follow-up period. Our results showed that, six months after surgery, recurrence was observed in three eyes (10.3%) in the mitomycin C group and seven eyes (20.6%) in the cyclosporine group, confirming that the two methods were effective in reducing the pterygium recurrence rate. In contrast, recurrence was observed in 15 eyes (41.7%) in the bevacizumab group, which did not differ from the artificial tears group (15 eyes, 45.5%); thus, bevacizumab did not affect recurrence rate (p = 0.004). However, at three months after surgery, recurrence was observed in only two eyes (5.6%) in the bevacizumab group, which was similar to findings in the mitomycin C and cyclosporine groups and consistent with previously reported results that bevacizumab has a limited effect in treating pterygium [922].
Although the half-life of 1.25 mg bevacizumab is about 9.8 days in the vitreous cavity [23], bevacizumab is thought to be absorbed more rapidly in the conjunctiva because of the abundance of blood vessels. Thus, it is unlikely that the effect of the 2.5% topical bevacizumab used in this study could last longer than one month, and recurrence rates are thought to have increased as soon as treatment was suspended after three months. In addition, unlike the majority of previous studies that used subconjunctival injections, we chose instillation four times a day for three months in order to prolong the effect. However, topical administration showed no marked differences compared to subconjunctival injection. Similar to previous studies, our results indicate that the use of bevacizumab to treat pterygium does not cause severe complications [922], even with instillation for three months instead of a single administration, with no particular complications other than subconjunctival hemorrhage. Thus, bevacizumab appears to be relatively safe to use.
The low recurrence rates using topical mitomycin C and topical cyclosporine are similar to previous results [218]. Although mitomycin C is effective in suppressing the recurrence of pterygium, various studies have focused on identifying the appropriate concentrations and duration of administration in order to avoid severe complications such as scleromalacia and corneal perforation. It is necessary to sufficiently inform patients about the possibility of severe complications and provide thorough training on instillation frequency and duration. Furthermore, previous studies have shown that compared to artificial tears or steroid drops, cyclosporine not only inhibits recurrence but also is associated with improvement in subjective symptoms, ocular surface disease index, tear film failure time, and Schirmer test, in terms of symptoms caused by pterygium such as hemorrhage, edema, and irritation [3]. However, Lee et al. [24] reported that exposing in vitro pterygium cells to 0.05% or lower concentrations of cyclosporine for fewer than 10 minutes did not suppress cell proliferation or cause severe cell damage; thus, the effect on recurrence is likely negligible. That study, however, was an in vitro experiment; given the environmental differences related to in vivo cell growth, further studies are required to confirm our findings.
Because each drug inhibits pterygium recurrence through different mechanisms [789], additional studies could use mixtures, different sequences, and combinations of these individual adjuvant therapies to evaluate their combined effectiveness at reducing the recurrence rate after pterygium surgery. Any such investigations would also have to carefully monitor for unexpected side effects and potential complications associated with each drug combination.
In the current work, to assess possible corneal endothelial toxicity and stability in each group, preoperative and postoperative corneal endothelial cell densities were measured and were not significantly different. We also note that the bare sclera method was used to perform pterygium excision, which usually has a higher recurrence rate than amniotic membrane transplantation or conjunctival autograft. Because this method is simple and relatively easy to perform, we thought that it would not be influenced by variability in surgical skill.
In conclusion, topical mitomycin C and topical cyclosporine treatments after pterygium surgery were effective in reducing the pterygium recurrence rate. Although topical bevacizumab was not effective in reducing recurrence, it did inhibit the proliferation of fibrovascular tissues in the short term. Additional studies should be performed using different doses and methods of administration with each adjuvant therapy and in combination, along with assessment of potential complications.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.