Comparison between Glaucomatous and Non-glaucomatous Eyes with Unilateral Retinal Vein Occlusion in the Fellow Eye
Article information
Abstract
Purpose
To evaluate and compare the clinical and angiographic characteristics of retinal vein occlusion (RVO) in glaucomatous and non-glaucomatous eyes with unilateral RVO in the fellow eye.
Methods
Twenty-one glaucomatous eyes (GL group) and 25 age-matched non-glaucomatous eyes (non-GL group) with unilateral RVO in the fellow eye were included in this study. Fluorescein angiographic images were assessed in both groups by 3 retina specialists in order to determine the RVO occlusion site. The occlusion site was divided into 2 types: arteriovenous (AV)-crossing and non-AV-crossing (optic cup or optic nerve sited). The clinical characteristics and prevalence of AV-crossing and non-AV-crossing RVO were compared between the 2 groups.
Results
The mean baseline intraocular pressures of the RVO eye and the fellow eye did not differ between the 2 groups (RVO eye: 14.3 ± 2.5 mmHg [non-GL group], 15.5 ± 3.9 mmHg [GL group], p = 0.217; fellow eye: 14.4 ± 2.5 mmHg [non-GL group], 15.7 ± 3.7 mmHg [GL group], p = 0.148). The prevalence of systemic disease did not differ between the 2 groups (e.g., diabetes mellitus and hypertension, p = 0.802 and 0.873, respectively). AV-crossing RVO was significantly more frequent in the non-GL group (19 eyes; 76%) than in the GL group (4 eyes, 19%, p < 0.001).
Conclusions
Non-AV-crossing RVO, i.e., optic cup- or optic nerve-sited RVO, is more frequently associated with glaucomatous changes in the fellow eye. Therefore, this type of RVO should be monitored more carefully for indications of glaucoma in the fellow eye.
Previously published studies have reported a correlation between retinal vein occlusion (RVO) and glaucoma [1-5]. In particular, the population-based Beaver Dam Eye Study found that the optic cup-to-disc ratio was a significant predictor of RVO [4]. The Blue Mountain Study reported that RVO was significantly associated with glaucoma, hypertension, stroke, and angina [5]. More recently, Kim et al. [3] showed that the thickness of the retinal nerve fiber layer (RNFL) was thinner in the fellow eyes of patients with unilateral RVO, especially in the infero- and superotemporal sectors, compared with control eyes.
True glaucomatous changes cannot be easily assessed in RVO-affected eyes because the retinal hemorrhage and edema generated by this disorder affect the states of the optic disc, RNFL, and visual field (VF). In patients with unilateral RVO, the fellow eye can serve as an appropriate surrogate for determining the relationship between glaucoma and RVO [3]. Because glaucoma is mostly a bilateral disease, the observation of structural glaucomatous changes in the fellow eye of patients with unilateral RVO suggests that these 2 disease entities (glaucoma and RVO) may share a common pathogenic mechanism. Additionally, the status of the fellow eye is important in cases of unilateral RVO, because if RVO-induced irreversible damage occurs, then only the fellow eye remains functional for sight. Thus, the possibility of glaucoma developing in the fellow eye of an RVO patient is of clinical importance in patient care.
Although the relationship between glaucoma and RVO is well recognized, not every patient with unilateral RVO shows glaucomatous structural changes in the fellow eye. Some (or many) eyes with unilateral RVO in the fellow eye do not demonstrate glaucomatous optic disc changes, although glaucomatous damage may be indicated in the future as this may be a cross-sectional observational effect. Therefore, we hypothesized that there might be some differences between glaucomatous and non-glaucomatous eyes with unilateral RVO in the fellow eye in terms of the baseline characteristics and RVO types. Hence, the aim of the present study was to compare the baseline characteristics of the patients and the fluorescein angiographic findings in RVO, especially in terms of the occlusion sites of RVO, between glaucomatous and non-glaucomatous eyes (with apparently healthy optic discs) that had unilateral RVO in the fellow eye.
Materials and Methods
Subjectstical methods
Subjects with unilateral RVO evaluated between 2009 and 2011 at the glaucoma and retina clinic of Asan Medical Center, Seoul, Korea and who met the criteria described below were consecutively included by retrospective medical record review.
Retinal specialists (JGK and JYL) diagnosed unilateral RVO using dilated fundus examination, fundus photography, and fluorescein angiography. At the initial examination, each participant included in this study received a comprehensive ophthalmologic examination, including a review of their medical history, measurement of best-corrected visual acuity (BCVA), slit-lamp biomicroscopy, Goldmann applanation tonometry, gonioscopy, dilated funduscopic examination using a 90- or 78-diopter (D) lens, stereoscopic optic disc photography, RNFL photography (AFC-210, Nidek Technologies Srl, Tokyo, Japan; TRC IX50, Topcon, Tokyo, Japan), and fluorescein angiography (TRC IX50, Topcon). VF testing (Humphrey field analyzer using the Swedish interactive threshold algorithm [SITA] 24-2; Carl Zeiss Meditec, Dublin, CA, USA) was performed if glaucoma was suspected.
For inclusion in the study, all participants had to meet the following criteria: unilateral RVO in the fellow eye; BCVA ≥ 20 / 30 with a spherical equivalent within ±5 D and cylinder correction within +3 D; and the presence of a normal anterior chamber and open-angle on slit-lamp and gonioscopic examinations.
Cases of unilateral RVO with confirmed glaucoma in the fellow eye were included in the 'glaucoma (GL) group.' Age-matched healthy eyes with unilateral RVO in the fellow eye were included in the 'non-glaucomatous (non-GL) group.' Glaucomatous eyes were diagnosed with glaucomatous VF defects that were confirmed by at least 2 reliable VF examinations and the presence of glaucomatous optic disc changes (e.g., increased cupping [>0.7], neuroretinal rim thinning, disc excavation, RNFL defects, and disc hemorrhage). Eyes with glaucomatous VF defects were defined as follows: those with a cluster of 3 points with probabilities <5% on the pattern deviation map in at least 1 hemifield, including at least 1 point with a probability <1%; a cluster of 2 points with a probability <1% and a glaucoma hemifield test result outside of the normal limits; or a pattern standard deviation outside 95% of the normal limits. Only reliable VF test results (false-positive errors <15%, false-negative errors <15%, and fixation loss <20%) were included in the analysis. Patients with other ophthalmic or neurologic conditions that could result in VF defects or who had undergone previous intraocular surgery (other than cataract extraction) were excluded.
All procedures conformed to the principles of the Declaration of Helsinki, and the study was approved by the Institutional Review Board of the Asan Medical Center at the University of Ulsan, Seoul, Korea.
Fluorescein angiographic evaluation
Fluorescein angiographic images were evaluated by 3 retina specialists (DHL, SGJ, and JTK). Images were collected and displayed on an LCD (liquid crystal display) monitor. The three graders independently assessed the images by determining the venous occlusion sites in the eyes affected by RVO using a modified version of the Beaumont and Kang classification [6]. Venous occlusion was classified as arteriovenous (AV)-crossing RVO (Fig. 1A), optic cup-sited RVO, or optic nerve-sited RVO. Optic cup- and optic nerve-sited RVO were combined into the single category of "non-AV-crossing RVO" (Fig. 2B) for the current analysis.
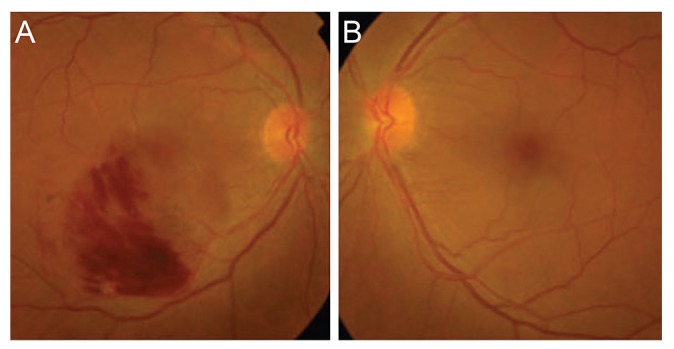
Clinical case of non-glaucomatous eye with unilateral retinal vein occlusion (RVO) in the fellow eye. This patient had arteriovenous crossing RVO in the right eye (A) and non-glaucomatous eye in the left (B).
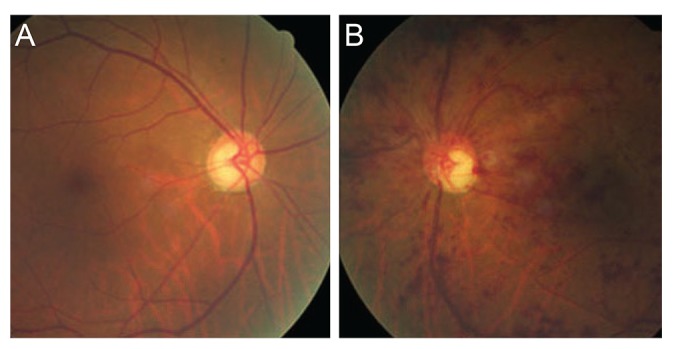
Clinical case of glaucomatous eye with unilateral retinal vein occlusion (RVO) in the fellow eye. This patient had glaucomatous eye in the right (A) and non-arteriovenous-crossing type RVO (B) in the left eye.
Each grader was masked to the results of the other graders and to all clinical information, including inclusion in the GL or non-GL group. The optic disc was removed in order to obviate any bias during the decision-making process. If the opinions of all 3 observers differed, then a decision was reached by consensus.
Statistical analysis
The baseline clinical characteristics were compared between the GL and non-GL groups. The Wilk-Shapiro test was used to determine the distribution of the numerical data. Normally distributed data are presented as the means and standard deviations. Non-normally distributed data are shown as the medians and interquartile ranges. Normally distributed data were compared between the GL and non-GL groups using the unpaired t-test. Non-normally distributed data were compared using the Mann-Whitney test. The chi-square test was used to compare categorical data.
The prevalence of AV- and non-AV-crossing RVO was compared between the 2 groups using the chi-square test. Statistical analyses were performed using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA).
Results
The participants in this study were patients with unilateral RVO without (25 patients, non-GL group) or with (21 patients, GL group) glaucoma in the fellow eye. The VF mean deviation in the glaucomatous eyes (fellow eye of RVO) was -8.07 decibels in the GL group. The mean ages of the non-GL and GL groups were not significantly different (60.4 ± 5.8 vs. 63.7 ± 10.4 years, respectively; p = 0.191), as expected. The mean baseline untreated intraocular pressure (IOP) of the RVO eye in the non-GL group was 14.4 ± 2.5 mmHg and 15.5 ± 3.9 mmHg in the GL group (p = 0.217). The mean baseline IOP of the fellow eye of the RVO in the non-GL group was 14.4 ± 2.5 mmHg and 15.7 ± 3.7 mmHg in the GL group (p = 0.148). The prevalence of systemic disease, such as diabetes mellitus (DM) or hypertension, did not differ between the 2 groups (p = 0.802 and 0.873, respectively) (Table 1).
AV-crossing RVO (19 eyes; 76.0%) was dominant in the non-GL group, whereas it was only found in 4 eyes (19.0%) in the GL group; thus, optic nerve- or optic cup-sited RVO was found in 17 eyes in the GL group. The prevalence of AV- and non-AV-type RVO differed significantly between the 2 groups (p < 0.001) (Table 2).

The prevalence of AV-crossing and non-AV-crossing retinal vein occlusion in GL and non-GL groups (chi-square test)
Representative clinical examples are shown in Figs. 1 (non-GL group) and 2 (GL group). Fig. 1 shows a man aged 58 years who had AV crossing RVO in the right eye (Fig. 1A) and non-GL eye in the left. Fig. 2 shows a 70-year-old man with glaucoma in the right eye (Fig. 2A) and non-AV-crossing RVO in the left eye (Fig. 2B).
Discussion
To evaluate our hypothesis that there might be some differences in terms of the clinical characteristics between glaucomatous and non-glaucomatous eyes with unilateral RVO in the fellow eye, we enrolled 2 groups of patients. The aim of this analysis was to compare the prevalence of systemic disease, such as DM and hypertension, untreated IOP at baseline, and the characteristics of RVO in terms of occlusion site between these 2 groups.
Research on clinical outcomes has indicated that RVO affects patients' clinical characteristics and RVO occlusion site [6]. Thus, the present study investigated the RVO occlusion sites of the 2 groups. Beaumont and Kang [6] defined 3 types of RVO: AV-crossing, optic cup-sited, and optic nerve-sited RVO, according to the occlusion site. They differentiated optic cup- and optic nerve-sited RVO by the presence of a narrowed papillary vein within the optic cup, which indicates optic cup-sited RVO; however, a dilated papillary vein suggests optic nerve-sited RVO. In some of our cases, this difference was not obvious because of swelling of the optic nerve or hemorrhage that obscured the contours of the papillary vein. Furthermore, our study included a limited sample size. Thus, we modified their criteria by classifying both optic cup- and optic nerve-sited RVO as non-AV-crossing RVO. Hence, the RVO occlusion site was categorized as 1 of 2 types, AV-crossing or non-AV-crossing, depending on its vicinity to the optic disc in the current analysis.
Our results indicated that most of the glaucomatous eyes (81%) had non-AV-crossing RVO in the fellow eye, while AV-crossing RVO was dominant in the fellow eye of the non-glaucomatous eyes. Therefore, among the various types of RVO, optic cup- or optic nerve-sited RVO seemed to be more frequently associated with glaucoma. This observation coincided with results reported by Beaumont and Kang [6]. They categorized the type of RVO according to occlusion site and found that optic cup-sited RVO demonstrated a higher prevalence in patients with glaucoma. Our observation may stem from a different pathogenic mechanism of RVO as to its occlusion site. Compression of the retinal vein by a thickened retinal arterial wall at the AV-crossing site is known to result in AV-crossing RVO. Moreover, high IOP is a risk factor for optic nerve- or optic cup-sited RVO [7].
Interestingly, our patients demonstrated no differences in terms of IOP between the 2 groups in either the RVO eye or the fellow eye. In other words, the mean baseline untreated IOP did not differ between non-glaucomatous and glaucomatous eyes. Furthermore, the IOP of the RVO eye did not differ between groups. Overall, the majority of patients with primary open-angle glaucoma were within the normal range for IOP in East Asian countries, such as Korea and Japan [8,9]. Kim et al. [3]'s study performed in Korea also showed similar levels of IOP between RVO eyes, which had decreased RNFL thickness in the fellow eye and healthy eyes. Therefore, optic nerve- and optic cup-sited RVO may be caused by the relative weakness of the lamina cribrosa, and not IOP itself. The lamina cribrosa is known as the occlusion site of optic nerve-sited RVO [10].
Systemic vascular diseases such as DM and hypertension are well recognized as important risks factors for the development of RVO [11-13]. To determine if systemic disease increases the risk of glaucoma in established RVO eyes, we compared the prevalence of DM and hypertension between the 2 groups. Our results indicated that the prevalence of hypertension and DM did not differ between the 2 groups. Therefore, our observations revealed that the systemic risk factors for RVO did not further increase the risk of developing glaucoma in RVO eyes. One of the strongest risk factors for glaucoma is age [14-16]. In order to reduce the confounding effects of age, we matched for age when selecting non-glaucomatous control eyes.
The relationship between glaucoma and RVO has been acknowledged in numerous publications [1-5], and clinicians often encounter patients who have both diseases at the same time in real practice. Therefore, the relationship between the 2 diseases has important implications for patient care, as well as for determining possible common pathogenic mechanisms, because the fellow eye in RVO is the only eye to retain sight function for patients if RVO causes irreversible damage. Hence, the fellow eye in RVO should be cautiously monitored for the possible development of glaucoma if the association between the 2 diseases is strong. Our results indicate that if the retinal vein is occluded at a non-AV-crossing site (i.e., optic cup- or optic nerve-sited), the fellow eye should be monitored more carefully.
The limitation of our study was a relatively small sample size. However, to the best of our knowledge, there have been no previous studies comparing glaucomatous and non-glaucomatous eyes with RVO in the fellow eye.
In conclusion, non-AV-crossing RVO is more frequently associated with glaucoma in the fellow eye, regardless of IOP. Therefore, we suggest that non-AV-crossing RVO be monitored more carefully for the possible development of glaucoma in the fellow eye.
Notes
No potential conflict of interest relevant to this article was reported.
