Effect of Age and Early Intervention with a Systemic Steroid, Intravenous Immunoglobulin or Amniotic Membrane Transplantation on the Ocular Outcomes of Patients with Stevens-Johnson Syndrome
Article information
Abstract
Purpose
This retrospective observational case series of fifty-one consecutive patients referred to the eye clinic with acute-stage Stevens-Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN) from 1995 to 2011 examines the effect of early treatment with a systemic corticosteroid or intravenous immunoglobulin (IVIG) on the ocular outcomes in patients with SJS or TEN.
Methods
All patients were classified by age (≤18 years vs. >18 years) and analyzed by treatment modality and early intervention with systemic corticosteroids (≤5 days), IVIG (≤6 days), or amniotic membrane graft transplantation (AMT) (≤15 days). The main outcomes were best-corrected visual acuity (BCVA) in logarithm of the minimum angle of resolution (logMAR) and ocular involvement scores (OIS, 0-12), which were calculated based on the presence of superficial punctate keratitis, epithelial defect, conjunctivalization, neovascularization, corneal opacity, keratinization, hyperemia, symblepharon, trichiasis, mucocutaneous junction involvement, meibomian gland involvement, and punctal damage.
Results
The mean logMAR and OIS scores at the initial visit were not significantly different in the pediatric group (logMAR = 0.44, OIS = 2.76, n = 17) or the adult group (logMAR = 0.60, OIS = 2.21, n = 34). At the final follow-up, the logMAR and OIS had improved significantly in the adult group (p = 0.0002, p = 0.023, respectively), but not in the pediatric group. Early intervention with IVIG or corticosteroids significantly improved the mean BCVA and OIS in the adult group (p = 0.043 and p = 0.024, respectively for IVIG; p = 0.002 and p = 0.034, respectively for corticosteroid). AMT was found to be associated with a significantly improved BCVA or OIS in the late treatment group or the group with a better initial OIS (p = 0.043 and p = 0.043, respectively for BCVA; p = 0.042 and p = 0.041, respectively for OIS).
Conclusions
Our findings suggest that patients with SJS or TEN who are aged 18 years or less have poorer ocular outcomes than older patients and that early treatment with steroid or immunoglobulin therapy improves ocular outcomes.
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are severe blistering diseases of the skin and mucous membranes that are mostly caused by adverse drug reactions. They have a low incidence rate but a high level of mortality [1,2]. SJS is defined by epidermal detachment of less than 10% of the body surface area (BSA) and TEN as detachment of more than 30% of the BSA [3,4]. Generally, both SJS and TEN are used as a diagnosis when the lesion involves between 10 and 30% of the skin. Ophthalmic involvement is common later in the disease, and in severe cases bilateral blinding due to corneal scarring and vascularization may occur [5-7].
Recent research indicates that immunologic activation of cytotoxic CD8 T cells, macrophages, IL-6, TNF-α, soluble Fas ligand (FasL), and others are involved in the pathogenesis of SJS and TEN [8-11]. Many studies have focused on treatments that modify the immunologic response, such as corticosteroids and intravenous immunoglobulin (IVIG), [12] in an effort to reduce the cytokine storm and Fas-FasL induced apoptosis. Amniotic membrane graft transplantation (AMT) may be an another option for diminishing ocular inflammation in the acute stage [13].
However, no standardized treatment has been established for SJS or TEN patients with eye involvement. Few studies have compared the effects of different treatments (such as oral steroids or IVIG) on ocular outcomes in the acute stage. Furthermore, according to our clinical experience, the effect of treatment on ocular inflammation tends to be age-dependent. Several studies have reported that prognosis (mortality and treatment outcome) differ between children and adults [4,14-16], but no study has addressed the effect of different treatments on the ocular outcomes in these groups.
Accordingly, the aim of the present study is to assess the effects of different treatments for SJS and TEN on ocular outcomes with respect to patient age.
Materials and Methods
The institutional review board of Seoul National University Hospital (SNUH) approved the study protocol (H-1110-112-383) and the protocol complied with the tenets of the Declaration of Helsinki.
Patients
In this retrospective observational case series, we examined the medical records of 51 consecutive patients who presented or were referred to the eye clinic at SNUH when they were in acute-stage SJS or TEN from 1995 to 2011. All medical records were obtained from the SNUH database and patients were identified based on the International Classification of Diseases 10th revision using the code L51.1 for SJS and L51.2 for TEN. All diagnoses were based on clinical history or biopsy results and patients were classified by the criteria outlined by Bastuji-Garin et al. [17].
Patients were excluded if there was no ophthalmic involvement during the acute stage, no follow-up visit at the eye clinic after the acute stage, no documentation by a corneal specialist, if disease onset occurred during corticosteroid use for control of another disease, or if there was a diagnosis of erythema multiforme major or minor.
Patients were classified by age (≤18 years vs. >18 years), treatment modality, time from onset of acute symptoms to treatment initiation (≤5 days for steroid, ≤6 days for immunoglobulin, or ≤15 days for AMT), and severity of ocular involvement at the initial visit (≤6 points of ocular involvement score [OIS] vs. >6 points of OIS, see below). Disease onset was defined as the day when the mucocutaneous lesion first developed.
Statistical methods
Statistical analysis was performed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). The Wilcoxon signed rank test and the Mann-Whitney U-test were used for continuous variables and Pearson's chi-square test or Fisher's exact test were used for categorical variables. Logistic regression analysis was used to calculate odds ratios. Statistical significance was accepted for p-values of <0.05.
Visual acuity and ocular involvement scores
Best-corrected visual acuity (BCVA) was tested at a distance of 5 m using the Snellen chart (Hahn's standard test chart; Hanil, Seoul, Korea) and results are presented as the logarithms of the minimal angle of resolution (logMAR) values.
All data on ocular involvement, which was documented by a corneal specialist, was reviewed and the OIS was calculated using criteria adapted from Sotozono et al. [18], with some modification. Briefly, OIS considers corneal complications (superficial punctate keratitis, epithelial defects, conjunctivalization, neovascularization, corneal opacity, and keratinization), conjunctival complications (hyperemia and symblepharon), and lid complications (trichiasis, mucocutaneous junction involvement, meibomian gland involvement, and punctal damage). The presence of each component was valued as 1 point and the sum (0 to 12 points) was used to indicate overall OIS.
Results
Patient characteristics
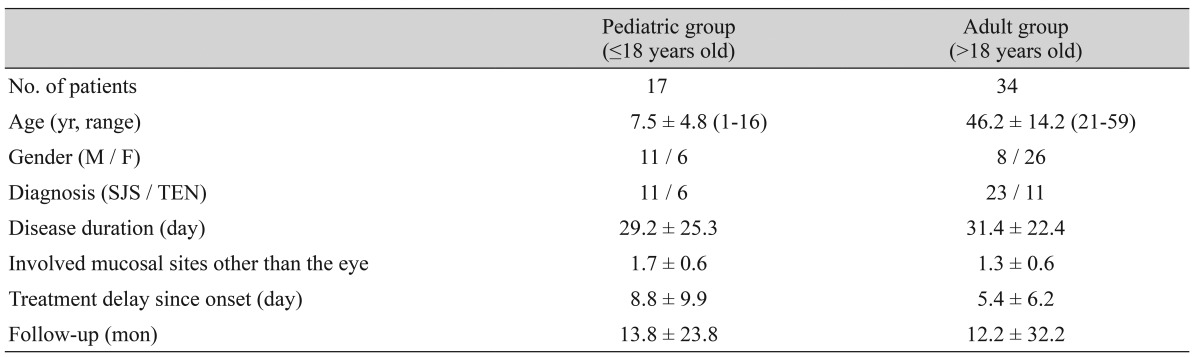
We first examined the demographic and clinical characteristics of the 51 enrolled patients (Table 1). Clinical outcomes of representative cases enrolled in this study are shown in Appendix 1. There was a significantly greater proportion of males in the pediatric group and females in the adult group (p = 0.004, Pearson's chi-square test). Pediatric patients had significantly more extraocular mucosal involvement (p = 0.008, Pearson's chi-square test). There were no other significant demographic differences between the groups.
Pediatric patients were more likely to be given IVIG while adult patients were more likely to be given systemic corticosteroid (p = 0.012 and 0.036, respectively; Fisher's exact test) (Table 2). Corticosteroids were administered at 2.93 ± 1.94 mg/kg/day (interquartile range, 0.75 to 5.09) for 3.50 ± 2.65 days (interquartile range, 1.25 to 6.25) in the younger group, and 5.28 ± 3.60 mg/kg/day (interquartile range, 3.33 to 5.50) for 3.47 ± 2.09 days (interquartile range, 2.00 to 4.00) in the older group. IVIGs were administered as 3.50 ± 1.52 g/kg/day (interquartile range, 2.00 to 4.50) for 4.33 ± 1.03 days (interquartile range, 3.75 to 5.25) in the younger group, and 2.67 ± 0.58 g/kg/day (interquartile range, 2.00 to 3.00) for 4.00 ± 1.00 days (interquartile range, 3.00 to 4.00) in the older group. In these two groups, treatment modality was not found to affect ocular outcomes when compared to supportive care only (as indicated by logMAR and OIS). However, IVIG and corticosteroid treatment tended to provide some benefit (Table 3). AMT was first performed in October, 2003 in this case series and cryopreserved amniotic membrane was grafted to the ocular surface to fully cover the lid margins and palpebral conjunctiva as described by other authors [19,20]. Supportive care performed in this case series includes careful monitoring of fluid balance, respiratory function, nutritional requirements, and appropriate wound care [21].
Visual acuities
Next, we evaluated whether an improvement in visual acuity could be achieved by intervention, and whether this visual benefit is dependent on the age or the time of treatment initiation within each age group. Mean logMAR values in the pediatric group were similar at initial and final visits (0.44 ± 0.28 vs. 0.41 ± 0.77; p = 0.310, Wilcoxon's signed rank test) (Fig. 1A). Analysis of subgroups of the pediatric patients with respect to treatment modality and time of treatment initiation also indicated no significant change in logMAR between initial and final visits (p > 0.05, Wilcoxon's signed rank test). In contrast, the mean logMAR of the adult patients improved significantly over the same period (0.60 ± 0.57 vs. 0.43 ± 0.84; p = 0.0002, Wilcoxon's signed rank test) (Fig. 1A). However, mean logMAR values at initial and final visits were not significantly different in each pediatric or adult groups (p > 0.05, Mann-Whitney U-test) (Fig. 1A).

Changes in visual acuities and ocular involvement score (OIS) with respect to patient age. (A) Mean logarithms of the minimal angle of resolution (logMAR) values in the pediatric group were similar at initial and final visits. In contrast, the mean logMAR of the adult patients improved significantly over the same period. (B) The pediatric group also showed no significant difference in mean OIS between initial and final visits. However, the adult group showed a significant improvement in mean OIS. There was no significant between group differences in each visits. Pediatric group = who are aged 18 years or less; adult group = who are over 18 years old. *Wilcoxon's signed rank test.
Early treatment of adult patients with IVIG (≤6 days) was found to be associated with a significantly improved logMAR at final visit (initial visit, 0.45 ± 0.41; final visit, 0.25 ± 0.66; p = 0.043, Wilcoxon's signed rank test) (Fig. 2). In addition, early treatment in adult patients with systemic corticosteroids (≤5 days) was associated with a significantly improved logMAR at final visit (initial visit, 0.41 ± 0.37; final visit, 0.34 ± 0.80; p = 0.002, Wilcoxon's signed rank test) (Fig. 2).
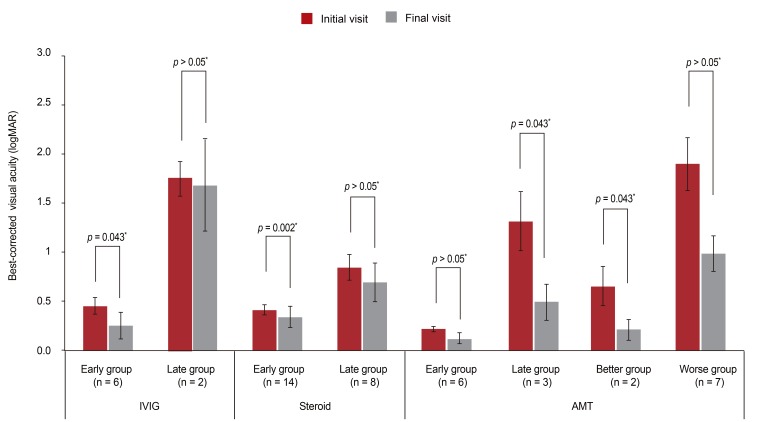
Changes in visual acuities of the adult group with respect to treatment modality and time of treatment initiation. Early treatment of adult patients with intravenous immunoglobulin (IVIG) or with systemic corticosteroids was found to be associated with a significantly improved logMAR at final visit. The mean logMAR of patients treated with amniotic membrane graft transplantation (AMT) improved significantly by the final visit if patients were treated 15 days after disease onset or if their ocular involvement score was less than 6 at the initial visit. There was no significant between group differences in each visits. Early group = patients with treatment initiation ≤6 days for IVIG, ≤5 days for corticosteroid, or ≤15 days for AMT. Late group = treatment initiation >6 days for IVIG, >5 days for corticosteroid, or >15 days for AMT. Better group = patients with ocular involvement scores (OIS) of less than 6 at initial visit. Worse group = patients with OIS over 6 at initial visit. *Wilcoxon's signed rank test.
All patients with OISs of less than 6 who were treated by AMT had significantly improved logMAR values (initial visit, 0.65 ± 0.56; final visit, 0.21 ± 0.29; p = 0.043, Wilcoxon's signed rank test) (Fig. 2). In addition, the mean logMAR of patients treated with AMT 15 days after disease onset improved significantly (1.31 ± 1.04 vs. 0.49 ± 0.62; p = 0.043, Wilcoxon's signed rank test) (Fig. 2).
Ocular involvement
Finally, we assessed whether ocular involvement is improved by intervention and whether improvements are dependent on age, ocular severity, or the timing or types of interventions. The pediatric group showed no significant difference in mean OIS between initial and final visits (p = 0.076, Wilcoxon's signed rank test) (Fig. 1B). However, the adult group showed a significant improvement in mean OIS (p = 0.023, Wilcoxon's signed rank test) (Fig. 1B). Comparison of individual components of the OIS in the pediatric and adult groups indicated no significant differences at initial and final visits (p > 0.05 for all, Fisher's exact test and chi-square test) (Fig. 1B).
Analysis of treatment modality and time of treatment onset in pediatric patients indicated no significant differences in mean OIS (p > 0.05 for both, Wilcoxon's signed rank test). However, adult patients treated early (≤6 days) with IVIG showed a significantly better mean OIS than those treated later (2.00 ± 1.13 vs. 1.24 ± 1.86; p = 0.024, Wilcoxon's signed rank test) (Fig. 3). In addition, adult treated early (≤5 days) with corticosteroids also showed a significantly better mean OIS (2.41 ± 1.00 vs. 1.44 ± 2.13; p = 0.034, Wilcoxon's signed rank test) (Fig. 3).

Changes in ocular involvement score (OIS) of the adult group with respect to treatment modality and time of treatment initiation. Early treatment of adult patients with intravenous immunoglobulin (IVIG) or with systemic corticosteroids was found to be associated with a significantly improved OIS at final visit. The mean OIS of patients who were treated with amniotic membrane graft transplantation (AMT) improved significantly in the late group and worse group. There was no significant between group differences in each visits. Early group = patients with treatment initiation ≤6 days for IVIG, ≤5 days for corticosteroid, or ≤15 days for AMT. Late group = treatment initiation >6 days for IVIG, >5 days for corticosteroid, or >15 days for AMT. Better group = patients with OIS of less than 6 at initial visit. Worse group = patients with OIS over 6 at initial visit. *Wilcoxon's signed rank test.
All patients with OISs of <6 who were given AMT treatment had a significantly better mean OIS than patients with OISs of >6 (4.00 ± 0.89 vs. 2.00 ± 1.55; p = 0.041, Wilcoxon's signed rank test) (Fig. 1B). Furthermore, OIS in all patients treated with AMT more than 15 days after disease onset improved significantly (5.29 ± 2.36 vs. 3.00 ± 2.52; p = 0.042, Wilcoxon's signed rank test) (Fig. 3).
Discussion
This study shows that pediatric patients with SJS or TEN under 18 years of age had poorer ocular outcomes, measured via OIS, than adult patients. Furthermore, our findings suggest that early treatment with corticosteroid or immunoglobulin therapy significantly improves ocular outcomes in adult patients. These findings are of importance because they provide information on relative prognoses based on patient age during the acute stage and because they prompt intervention with systemic anti-inflammatory drugs during the acute stage, which might beneficially modify the ocular course of disease.
SJS and TEN are characterized by marked keratinocyte apoptosis in the epidermis, dermo-epidermal separation, and overall epidermal necrosis [22]. Although their pathogeneses are not fully understood, several lines of evidence indicate that disruption of the immune system is involved. Viard et al. [11] showed that soluble FasL is elevated in patients with SJS or TEN and that the expression of FasL is upregulated on keratinocytes. Abe et al. [8] found that the peripheral blood mononuclear cells of SJS patients secrete soluble FasL when exposed to drugs. Others have shown that massive apoptosis of keratinocytes is induced by perforin or granzyme B, which are released by drug-specific cytotoxic T cells [10,23]. Granulysin is secreted by activated CD8+ T cells, NK cells, and NKT cells, and also appears to be involved in keratinocyte apoptosis [9]. Based on the pathogeneses of SJS and TEN, it could be assumed that systemic steroid or IVIG therapy might reduce acute stage inflammation.
Systemic corticosteroids, which have well-known anti-inflammatory and immunosuppressive effects, have long been used to treat SJS and TEN. Multiple mechanisms have been reported to be involved in immunosuppression, including the stabilization of lysosomal membranes, the suppression of prostaglandin synthesis, the inhibition of the transcriptions of pro-inflammatory cytokines (IL-1, IL-2, IL-6, IFN-γ, and TNF-α), impairments in monocyte and macrophage function, and reductions in the numbers of circulating CD4+ T cells. However, there is little clinical evidence of their efficacy in reducing ocular disease during acute stage SJS or TEN. Anecdotal reports indicate a beneficial effect for systemic corticosteroid treatment, but study populations were small [24,25]. On the other hand, others have reported no benefit for systemic corticosteroid treatment [6]. Accordingly, more studies are needed to determine whether systemic steroids reduce ocular inflammation in SJS and TEN patients. In the present study, we found that visual acuity and ocular involvement significantly improved after instituting systemic corticosteroids, especially when administered within 5 days of acute symptom onset. That is, the present study supports previous reports regarding the beneficial effects of steroids in SJS and TEN. Furthermore, previous studies and the present study support the notion that the control of severe inflammation as early as possible is associated with fewer ocular sequelae. Inflammation is believed to be a principal pathogenic factor in limbal stem cell deficiency, which manifests clinically as a loss of palisades of Vogt, keratinization, and opacity in chronic-stage SJS or TEN [18]. Inflammation is also associated with loss of goblet cells in conjunctivae, which could result in severely dry eyes and ocular surface complications [5,6]. Furthermore, in the present study early treatment appeared to save some cells from total destruction, and thus, reduced ocular surface damage. On the other hand, systemic corticosteroid treatment could lead to numerous adverse effects, including gastrointestinal bleeding, which is accompanied by a high risk of mortality [26,27]. The more BSA involved, the higher the risk of mortality. Generally, the risk is considered serious in patients with body surface area involvement of more than 30% [28,29]. Therefore, close monitoring is recommended in patients being treated with systemic steroids when BSA involvement exceeds this level.
IVIG has also been used to treat SJS and TEN based on the belief that these antibodies prevent keratinocyte apoptosis resulting from Fas-FasL interaction [11]. However, the therapeutic efficacy and outcome of IVIG treatment remains controversial and no definitive study has addressed the effect of IVIG on ocular outcome. In the present study, we found that IVIG led to significant improvements in visual acuity and ocular involvement, especially when it was administered within 6 days of disease onset. Considering that the level of FasL increases several days before the onset of clinical manifestations, and decreases rapidly to reach the normal range at 5 days after disease onset in patients with SJS or TEN [30], early IVIG treatment may provide relief by saturating the Fas (CD95) binding site. Furthermore, the high frequency of infectious complications associated with corticosteroid treatment may be reduced by IVIG, which has anti-infectious and immunomodulatory properties [31,32]. In addition, IVIG could help to prevention of fluid loss, a significant problem in SJS and TEN, because of the osmolal effects of the proteins themselves [12]. Contrary to our results, Yip et al. [33] concluded that IVIG did not reduce the severity of ocular complications in patients with SJS or TEN. However, they enrolled a small number of patients, and only one patient was treated with IVIG alone; other patients were treated with IVIG either before or after steroid treatment.
We examined the effect of AMT during acute stage SJS and TEN. John et al. [19] initially reported that AMT was an effective treatment in patients with acute-stage TEN, and in subsequent studies found that AMT provides ocular benefits in patients with acute-stage SJS or TEN [20,34,35]. Recently, Gregory [13] found that AMT performed during the 10 days following disease onset reduced the risks of ocular sequelae. In the present study, we found that AMT significantly improved visual acuity and ocular outcome in patients with less severe ocular involvement (OIS <6), which suggests that AMT alone is insufficient to attenuate ocular inflammation in severe cases. Further study on the effects of AMT in SJS and TEN is necessary.
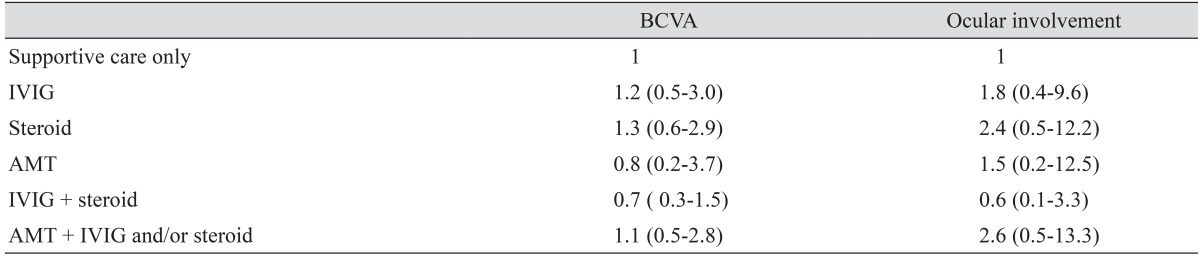
Interestingly, we found corticosteroid and IVIG provided benefits when compared with conservative treatment. The odds ratios for improvements in visual acuity and ocular involvement in adult patients treated with corticosteriods were 1.2 and 1.8, respectively, and 1.3 and 2.4, respectively, for adult patients treated with IVIG. Although these findings were not statistically significant, presumably due to the small sizes of the conservative treatment subgroup, they still provide a clue of possible benefit.
Another noteworthy finding of the present study was that prognosis appeared to be dependent on age. In particular, our results indicate that children given different treatments showed no significant improvements in visual acuity or ocular involvement relative to patients given best supportive care. This finding stands in contrast with those of several other reports that concluded SJS and TEN are milder and associated with lower mortality in children. However, these findings do concur with a recent study, in which higher long-term complications, including ocular sequelae, were reported in children compared with adults with SJS or TEN [14]. Taken together, it appears that ocular prognoses and treatment outcomes of children are quite different from those of adults. Further larger-scale investigations are warranted on this issue.
The main limitation of this study was that it did not have a prospective, randomized, controlled design, which would not be ethically tolerable in practice. Given the disease severities involved, we are satisfied with the retrospective design adopted. Second, some patients followed long-term by several examiners were included in the present study, and thus inter-individual measurement bias of ocular status was not fully excluded. Third, the small numbers of patients involved in the subgroup analyses of AMT and IVIG treatments might have affected our results.
In conclusion, our study indicates that early treatment with corticosteroid or immunoglobulin improves ocular outcomes in elderly patients, and suggests that patients aged 18 years or less with SJS or TEN have poorer ocular outcomes than adult patients.
Notes
Presented in part at the annual meeting of Korean Ophthalmological Society, Goyang, Korea, November 2011 and the American Society of Cataract and Refractive Surgery Symposium on Cataract, Intraocular Lens, and Refractive Surgery, Chicago, USA, April 2012.
No potential conflict of interest relevant to this article was reported.
Appendix
Appendix 1
Clinical outcomes of representative cases enrolled in this study. (A-C) Gross and slitlamp photographs of 7-year-old girl with Stevens-Johnson syndrome at initial visit (A,B) and last visit (C). (D-F) Slitlamp photograph of a 59-year-old woman with toxic epidermal necrolysis at initial visit (D,E) and at last visit (F). (G-I) 31-Year-old man with Stevens-Johnson syndrome at initial visit (G) underwent amniotic membrane graft transplantation (H), which resulted in clinical improvement at last visit (I).