|
 |
| Korean J Ophthalmol > Volume 27(5); 2013 > Article |
Abstract
Purpose
To comparatively analyze the methodological efficacy of the polymerase chain reaction (PCR) assay for herpes simplex virus 1 (HSV) detection in tears.
Methods
This retrospective study reviewed the medical records of 115 patients who were clinically diagnosed with herpes keratitis, and their tear samples were collected for HSV detection. PCR positive rates were analyzed for their dependence on the PCR primers used (conventional PCR primer vs. nested PCR primer), the tear collecting method used (micropipetting vs. collection with schirmer strip), the disease manifestation and the patient's previous medication history.
Results
HSV DNA was detected in 23 out of 115 (20%) tear samples. The PCR positive rate in tear samples did not differ depending on the PCR primer or tear collection method used. Typical epithelial lesions showed a higher positive rate (31.4%) than atypical epithelial lesions (10.9%). The previous history of the antiviral agent seemed to affect the PCR positive rate.
Herpes simplex virus 1 (HSV) keratitis has been one of the major causes of keratoplasty in South Korea [1]. A rapid, accurate diagnosis and immediate treatment using antiviral medication are critical to preventing corneal blindness. However, the clinical manifestation of herpes keratitis is too varied to be diagnosed solely based on its clinical findings. Up until now, laboratory diagnostic tools have not been good enough to detect HSV definitively in patients with herpes keratitis.
Several laboratory tests have been introduced, such as virus culture for virus isolation, immunofluorescence and polymerase chain reaction (PCR) assay to detect HSV. Recently, herpes PCR has been reported to have the advantages of higher sensitivity and shorter processing time than direct virus isolation as a standard procedure [2-5]. Nevertheless, PCR has some shortcomings that include altered results depending on the primer composition for the target DNA, the proficiency of the clinical laboratory worker and the risk of contamination.
The clinical feasibility of herpes PCR is still being debated in the ophthalmologic field because of the reported disadvantages mentioned above as well as more recent reports that have shown low sensitivity of herpes PCR in tears and epithelial cells. Therefore, the purpose of this study was to comparatively investigate the methodological efficacy of PCR assay in the detection of HSV in tears and to analyze other factors that affect the positive rate.
The medical records of a total 115 patients, who were clinically diagnosed as herpes keratitis and whose tear samples were collected for HSV detection, were retrospectively reviewed. The institutional review board of the Seoul National University Hospital approved this study's protocol (H-1201-015-392) and the protocol complied with the tenets of the Declaration of Helsinki. The patients who visited the Seoul National University Hospital during the period of November 2006 through July 2011 were enrolled in this study. The patients who had combined bacterial infections or other corneal degenerative or immune-related keratitis and who has less than six months of follow-up were excluded.
Two collection techniques for tear samples were employed. From November 2006 through March 2010, tears were collected from the lower fornix using schirmer strips (Eagle Vision, Memphis, TN, USA) for five minutes in patients who visited the clinic; this method had been adopted in a previous study [6]. After March 2010, tears were micropipetted, after 100 ┬Ąl of irrigation using normal saline in the lower fornix; a method that has been described in other study [7]. Both types of specimens were placed into a Tris-EDTA buffer and stored at -70Ōäā for HSV PCR assay.
DNA was extracted from the 50 ┬ĄL tear samples using the Magna Pure 96 system (Roche Applied Science, Indianapolis, IN, USA), as per the protocol from the manufacturer. This DNA extraction removed any residual fluorescein that could interfere with the PCR assay. A concentrated DNA sample of 0.01 mLwas used for PCR assay. From November 2006 to December 2008, simple PCR assay had been performed in the Department of Clinical Microbiology and Pathology at the Seoul National University Hospital. Oligonucleotide primers for HSV were designed to bracket a well-conserved region in the DNA polymerase gene of herpes simplex viruses. Primer pair HSV-P1 (5'-CGACTTTGCCAGCCTGTACC-3') and P2 (5'-AGTCCGTGTCCCCGTA-GATG-3') was used to amplify the locus of the DNA polymerase gene of HSV-1 and HSV-2. The DNA sample with 10 pmol of each primer pair contained 50 mM KCl, 10 nM Tris-HCl, 1.5 mM MgCl2, 0.01% gelatin, 5% dimethylsulfoxide, 200 ┬ĄM of each deoxynycleotide triphosphates, and 2.5 U of Taq polymerase (Perkin-Elmer Centus, Norwalk, CT, USA). The reactions were performed in an automated thermal cycler. The PCR cycle used was 1 minute at 94Ōäā as a denaturation step, 1 minute at 60Ōäā as an annealing step and 1 minute at 72Ōäā as a synthesis step, and was repeated 40 times [8]. Since January 2009, nested PCR assay had been used to detect HSV, using the HSV-1/HSV-2 oligomix Alert Kit (Nanogen Advanced Diagnostics, Corso Torino, Italy), which was optimized to amplify 160bp HSV-1 and 81bp HSV-2, according to the manufacturer's standard PCR condition and protocol in the department of clinical microbiology and pathology at the Seoul national University Hospital.
The 10 ┬ĄL of each amplified PCR product was loaded on a 2% agarose gel containing ethidium bromide (FMC Bioproducts, Rockland, ME, USA). These were then electrophoresed horizontally and each HSV DNA band was identified under ultraviolet transillumination.
The statistical comparison of PCR results was analyzed using the SPSS ver. 19 (IBM SPSS, Armonk, NY, USA). The chi-square test was used to compare the incidence of the positive result of PCR assay, to the two sampling methods, the two PCR assay methods, typical or atypical epithelial lesions of HSV keratitis, previous history of HSV keratitis and previous use of antiviral medication. Significance was assigned to calculated p-values <0.05.
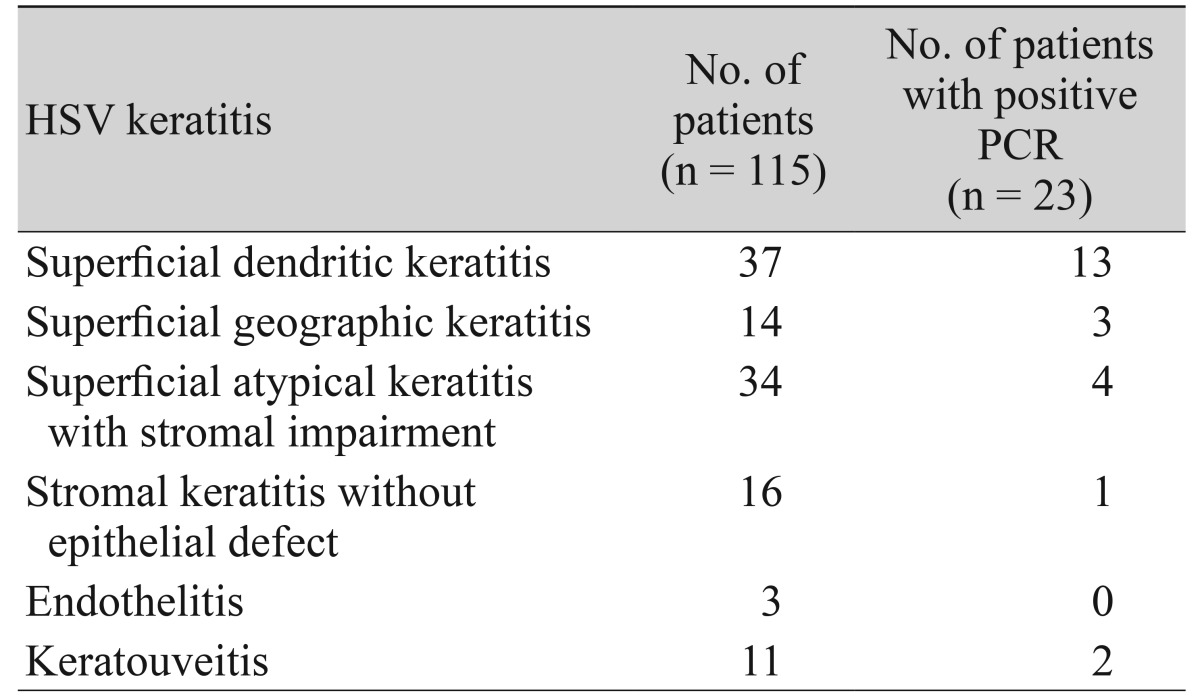
First, we analyzed the demographics of the enrolled patients. The mean age of the patients was 54.2 ┬▒ 19.2 years. The male to female ratio was 63 : 52. The mean follow up duration was 18.6 ┬▒ 7.9 months. Thirty-seven (33%) patients revealed a previous history of HSV keratitis on the same eye, and 55 patients had used the antiviral agent within 1 month of the tear sampling. Finally, 51 patients showed a typical dendritic or geographic epithelial lesion in their cornea (Table 1).
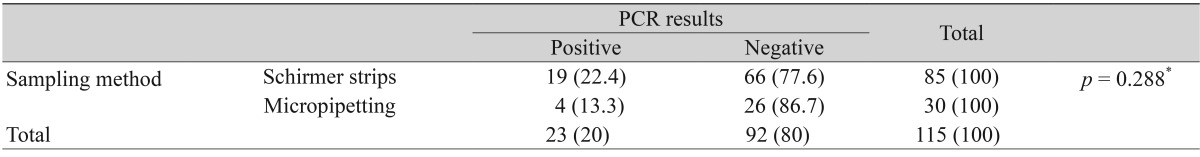
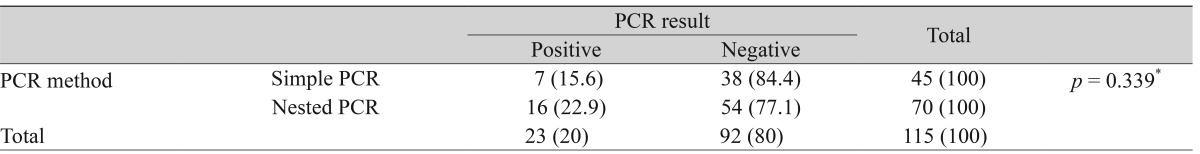
Next, we analyzed the total positive rate of tear PCR assay and the conditional positive rates according to the methods used to collect the tears and the methods used to detect herpes. The total incidence rate of positive PCR was 20% (23 out of 115) in the tears. There was no statistical difference in the positive PCR rates according to the tear collection method, i.e. use of the schirmer strip vs. micropipetting with normal saline irrigation (Table 2), or the PCR assay method, i.e. simple PCR and nested PCR (Table 3).
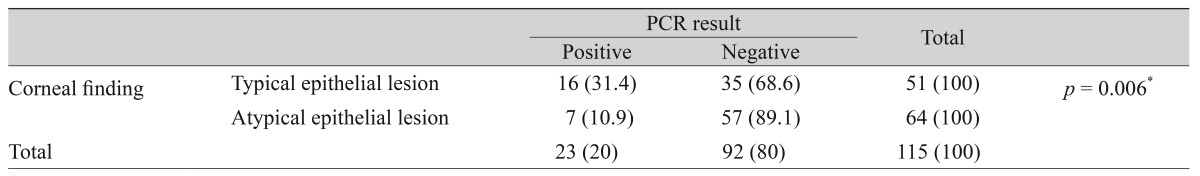
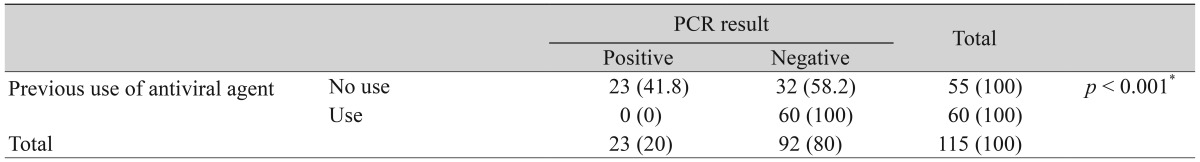
Finally, we investigated whether clinical factors affect the positive rates of tear PCR. The patients who presented with a typical corneal epithelial lesion displayed higher positive rates of PCR than the patients with an atypical epithelial lesion (p = 0.006) (Table 4). There was no significant statistical difference in the positive PCR rates between the patients with previous herpes keratitis history and patients without previous history (Table 5). Finally, the positive PCR rates were greater in the patients without previous usage of anti-herpecidal medication than in the patients with previous usage of the antiviral agent within 1 month before the tear sampling (p < 0.001) (Table 6).
This study found out that the positivity of tear PCR seemed to not be dependent on the tear collection method or the primers used. Because of its relatively low positive rate, HSV detection in tears using PCR was shown to be a supplementary diagnostic test in typical as well as atypical herpes epithelitis. In addition, previous usage of the anti-herpes medication appeared to affect the detection of herpes using PCR.
Since the sensitivity of PCR assays for the detection of the DNA of infectious microorganisms has been shown to be very high, which indicates that this method is an effective and valuable laboratory tool, continuous investigation regarding the feasibility of PCR to detect the herpes virus h as been performed [9,10]. In 1990, the first report came out that HSV DNA was detected in the cornea by PCR assay [11]. Thereafter, reports with small numbers of the cases were documented and these suggested that PCR is highly sensitive to herpes keratitis in tear and corneal scraping samples [12,13].
On the contrary, the studies with large numbers of cases did not show high sensitivity of PCR using tear or corneal scraping samples. Farhatullah et al. [14] reported a positive detection rate of HSV DNA in 49 out of 146 (33.6%) ocular samples using multiplex PCR. Satpathy et al. [6] presented that HSV DNA was detected in 32 out of 229 (13.97%) tear samples and in 56 out of 153 (36.66%) corneal scraping samples from suspected HSV keratitis patients with PCR assay. Finally, Hlinomazova et al. [15] showed a 40.09% HSV detection rate in 212 samples collected by flocked swabs using the real time PCR. Our data, which consisted of large numbers, also supported these studies, implying that tear PCR has a supplementary role in the diagnosis of herpes keratitis and should therefore not be used as a definitive tool.
This study was also consistent with Heo's preliminary report [16], which presented a lower detection rate of HSV DNA in tear specimens (4 out of 21, 19%) than in corneal scraping specimens (4 out of 6, 67%) in Korea. These data show that corneal scraping sampling was more sensitive to HSV DNA compared to tear sampling [6,16]. However, non-invasive tear samplings might be needed for PCR assays in cases where a corneal scraping sample could not be collected due to a thinned cornea, the lack of an epithelial lesion or poor compliance of the patients.
Additionally, this study attempted to find methods of increasing the sensitivity of the tear PCR method to HSV detection. For this reason, we compared the sensitivities of the conventional PCR primers, which have been used previously [12], and the nested PCR primers. The nested PCR is a two-step PCR with two pairs of PCR primers for a single locus to prevent unexpectedly primed PCR and improve diagnostic sensitivity. There have been several studies reporting that nested PCR was more sensitive than simple PCR [17-19]. However, in this study, there was no significant difference in the sensitivity between the simple PCR group and the nested PCR group. In addition, the tear collection method did not affect the sensitivity of HSV DNA detection. Considering that tears are constantly circulated, it is possible that tears, which stay in the eye until the very moment of the examination, may not hold enough HSV DNA to be detected consistently, regardless of the PCR primers or collection methods used.
Clinical manifestation, however, seemed to be involved in the detection rate of the HSV DNA in tear PCR. Our results showed a higher detection rate of HSV DNA in patients with typical epithelial lesions than in patients with atypical epithelial lesions or stromal keratitis. Our data support the results from a previous study that PCR assay detected fewer incidences of HSV DNA in the ocular specimens from the patients with HSV keratitis who presented with atypical epithelial lesions, or only stromal lesion, than patients who presented with typical epithelitis [16]. These results could be explained by a few hypotheses. First, atypical keratitis was detected at the late stage of the disease when HSV DNA was almost cleared from the tear and corneal surface by immune reactions, while typical epithelial lesions appeared at an early stage when viral replication was very active. The other possibility is misdiagnosis as atypical HSV keratitis, accompanied by no shedding of the HSV DNA from the stromal lesion to the tear in stromal keratitis. Nevertheless, the positive PCR may be used as a clue to determine the disease in the difficult cases of patients with atypical lesions, considering that tear PCR assay is a noninvasive supplementary tool that could help to make a rapid clinical diagnosis.
Finally, clinical treatment appeared to decrease the detection rate of HSV DNA in tear PCR. Systemic and topical antiviral agents, such as acyclovir or valaciclovir, which had a better oral bioavailability than acyclovir, have been commonly used to treat and prevent the HSV keratitis. They could effectively suppress the replication of HSV by inhibiting viral DNA polymerase in the ocular tissue. In addition to a previous report presenting that HSV DNA had a lower detection rate in tear samples of the HSV keratitis with pre-antiviral medication (3 out of 18, 16.66%) than in those without pre-antiviral medication (3 out of 3, 100%) [16], this study implied that treatment with antiviral medication could attenuate the detection rate of PCR because our data showed an increased detection rate of the HSV DNA by 41.8% (23 out of 55) in patients without any medication. This suggested that PCR assay would not help to make a diagnosis of HSV keratitis, if suspected patients had been given the antiviral medication recently before the sampling.
Our study was limited by the fact that it was not conducted as a non-randomized, controlled study in order to compare the sensitivity of each method. Furthermore, a considerable portion of the patients had been referred to our cornea clinic from other hospitals and had been treated with anti-viral medication, which has been shown to affect the detection rate. Finally, we could not perform viral culture or immunofluorescence detection of HSV in order to increase the specificity of the detection rate in tear PCR, due to time limitations and faculty shortage at the clinic. Nevertheless, this study provides relevant clinical information regarding tear herpes PCR and supports previous studies that indicated the supplementary role of tear PCR.
In conclusion, tear PCR assay seemed to be a supplementary laboratory test to diagnose HSV keratitis, and it might be affected by previous anti-viral treatment.
REFERENCES
1. Kim MK, Lee JH. Long-term outcome of graft rejection after penetrating keratoplasty. J Korean Ophthalmol Soc 1997;38:1553-1560.
2. Kaye SB, Baker K, Bonshek R, et al. Human herpesviruses in the cornea. Br J Ophthalmol 2000;84:563-571.



3. Khodadoost MA, Sabahi F, Behroz MJ, et al. Study of a polymerase chain reaction-based method for detection of herpes simplex virus type 1 DNA among Iranian patients with ocular herpetic keratitis infection. Jpn J Ophthalmol 2004;48:328-332.


4. Kowalski RP, Thompson PP, Cronin TH. Cell culture isolation can miss the laboratory diagnosis of HSV ocular infection. Int J Ophthalmol 2010;3:164-167.


5. El-Aal AM, El Sayed M, Mohammed E, et al. Evaluation of herpes simplex detection in corneal scrapings by three molecular methods. Curr Microbiol 2006;52:379-382.


6. Satpathy G, Mishra AK, Tandon R, et al. Evaluation of tear samples for herpes simplex virus 1 (HSV) detection in suspected cases of viral keratitis using PCR assay and conventional laboratory diagnostic tools. Br J Ophthalmol 2011;95:415-418.


7. Markoulli M, Papas E, Petznick A, Holden B. Validation of the flush method as an alternative to basal or reflex tear collection. Curr Eye Res 2011;36:198-207.


8. Rozenberg F, Lebon P. Amplification and characterization of herpesvirus DNA in cerebrospinal fluid from patients with acute encephalitis. J Clin Microbiol 1991;29:2412-2417.



9. Fox GM, Crouse CA, Chuang EL, et al. Detection of herpesvirus DNA in vitreous and aqueous specimens by the polymerase chain reaction. Arch Ophthalmol 1991;109:266-271.


10. Boerman RH, Arnoldus EP, Raap AK, et al. Polymerase chain reaction and viral culture techniques to detect HSV in small volumes of cerebrospinal fluid: an experimental mouse encephalitis study. J Virol Methods 1989;25:189-197.


11. Crouse CA, Pflugfelder SC, Pereira I, et al. Detection of herpes viral genomes in normal and diseased corneal epithelium. Curr Eye Res 1990;9:569-581.


12. Koizumi N, Nishida K, Adachi W, et al. Detection of herpes simplex virus DNA in atypical epithelial keratitis using polymerase chain reaction. Br J Ophthalmol 1999;83:957-960.



13. Yamamoto S, Shimomura Y, Kinoshita S, et al. Detection of herpes simplex virus DNA in human tear film by the polymerase chain reaction. Am J Ophthalmol 1994;117:160-163.


14. Farhatullah S, Kaza S, Athmanathan S, et al. Diagnosis of herpes simplex virus-1 keratitis using Giemsa stain, immunofluorescence assay, and polymerase chain reaction assay on corneal scrapings. Br J Ophthalmol 2004;88:142-144.



15. Hlinomazova Z, Loukotova V, Horackova M, Sery O. The treatment of HSV1 ocular infections using quantitative real-time PCR results. Acta Ophthalmol 2012;90:456-460.


16. Heo JY, Kim SJ, Kim JC, Hahn TW. The early diagnosis of herpetic keratitis by polymerase chain reaction. J Korean Ophthalmol Soc 2001;42:36-42.
17. Welch D, Lee CH, Larsen SH. Detection of plasmid DNA from all Chlamydia trachomatis serovars with a two-step polymerase chain reaction. Appl Environ Microbiol 1990;56:2494-2498.



18. Persing DH, Smith TF, Tenover FC, et al. Diagnostic molecular microbiology: principles and application. Washington, DC: American Society for Microbiology; 1993. p. 51-87.
19. Choi MY, Yoo JW, Choi TY, Kim YT. A study on the detection of herpes simplex virus using nested PCR. Korean J Clin Pathol 1997;17:764-771.
Table┬Ā1
Classification of HSV keratitis patients according to corneal lesion, and their tear PCR results
Table┬Ā2
The incidence of positive PCR results between schirmer strip sampling and inferior forniceal sampling using a micropipette
Table┬Ā4
The incidence of positive PCR results between patients with typical epithelial lesions and patients with atypical epithelial or stromal lesion only
- TOOLS




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print