Achromobacter xylosoxidans Keratitis after Contact Lens Usage
Article information
Abstract
To report on Achromobacter xylosoxidans keratitis in two healthy patients who had worn contact lenses foran extended period of time. A 36-year-old female and a 21-year-old female visited our hospital with ocular pain and blurred vision. Both patients had a history of wearing soft contact lenses for over fve years with occasional overnight wear. At the initial presentation, a slit lamp examination revealed corneal stromal infiltrations and epithelial defects with peripheral neovascularization in both patients. Microbiological examinations were performed from samples of corneal scrapings, contact lenses, contact lens cases, and solution. The culture resulting from the samples taken from the contact lenses, contact lens cases, and solution were all positive for Achromobacter xylosoxidans. Confrming that the direct cause of the keratitis was the contact lenses, the frst patient was prescribed ceftazidime and amikacin drops sensitive to Achromobacter xylosoxidans. The second patient was treated with 0.3% gatifoxacin and fortifed tobramycin drops. After treatment, the corneal epithelial defects were completely healed, and subepithelial corneal opacity was observed. Two cases of Achromobacter xylosoxidans keratitis were reported in healthy young females who wore soft contact lenses. Achromobacter xylosoxidans should be considered a rare but potentially harmful pathogen for lens-induced keratitis in healthy hosts.
Achromobacter xylosoxidans is a gram-negative bacillus that belongs to the genus Alicaligenes. It is often confused with Pseudomonas aeruginosa, a Gram-negative bacillus that is commonly identified in an isolation process and is also called xylosoxidans due to its ability to oxidize xylose. Achromobacter xylosoxidans can exist in any contaminated place, though it is mainly found in soil and water. It rarely causes infection in humans but has been known to cause opportunistic infections in patients with tumors, hematologic disease, organ transplants, hypogammaglobulinemia, or acquired immunodeficiency syndrome (AIDS) [1]. Since Achromobacter sepsis from a diagnostic apparatus was reported to have occurred in five patients at a Pennsylvania University Hospital in 1972, Achromobacter has drawn attention as a causative pathogen of nosocomial infection and for its reported resistance to various antibiotics [2-5]. In addition to sepsis, Achromobacter has also been reported to cause various infections such as meningitis, pneumonia, urinary duct disease, and peritonitis. There have recently been several reports on extraocular infection caused by Achromobacter xylosoxidans. These infections occurred in patients with suppressed corneal immunity, such as patients with corneal transplantation or endophthalmitis. In this paper, we report two cases of Achromobacter xylosoxidans keratitis in healthy young women who had worn soft contact lenses for an extended period of time.
Case Reports
Case 1
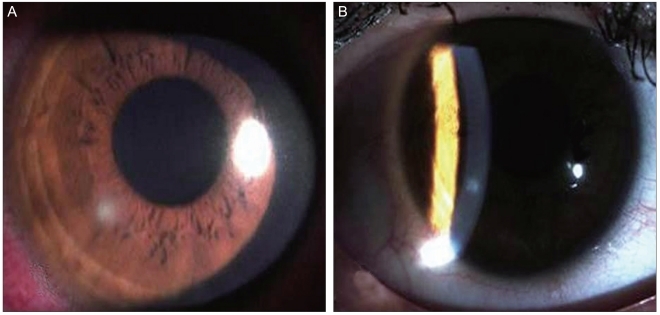
A 36-year-old female patient visited our hospital complaining of right ocular pain and reduced vision for the week before the visit. She had been diagnosed with right ocular keratitis in an ophthalmic clinic and took levofloxacin (Cravit; Santen, Osaka, Japan) for a week. She had been wearing soft contact lenses for 20 years, had suffered from keratitis caused by the contact lenses, and had been treated several times. She had no medical history of ocular injury, surgery, or treatment with ophthalmic or systemic steroids. No abnormal finding was observed in the blood test. The patient's corrected visual acuity was 20 / 20 in both eyes, and her intraocular pressure was 11 mmHg in the right eye and 16 mmHg in the left eye. Her right bulbar conjunctiva near the lesion had hyperemia, and a 0.5 × 0.7 mm corneal epithelial defect was seen with intra-stromal infiltration and peripheral neovascularization toward the 12 o'clock direction of the limbus (Fig. 1A). There were no abnormal findings in the lens, vitreous body, or retina. A bacteriological test was conducted on the corneal scrapings, contact lenses, lens cases, and lens washing agent. The right corneal ulcer was treated topically with gatifloxacin (Gatiflo; Seoul, Korea) with an initial one-hour interval and then a late reduced frequency; with 2% homatropine (Ocuhomapine Eye; Samil, Seoul, Korea) three times a day; and with 0.3% unpreserved hyaluronic acid (Hyalein mini, Santen).
(A) The slit-lamp photograph at the first visit revealed a 0.5 × 0.7 mm-sized round corneal epithelial defect with stromal infiltration. (B) Seven days after treatment, the corneal lesion exhibited complete epithelialization with subepithelial corneal opacity.
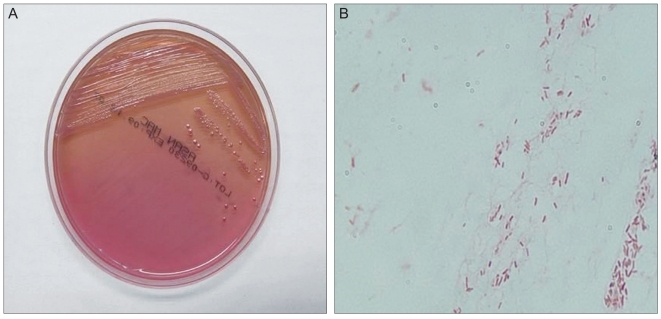
Improved symptoms and corneal status were initially observed. However, the patient visited the hospital again three weeks later due to deteriorating symptoms. The size of the corneal epithelial defect increased to 2.0 × 3.2 mm at the location of the previous lesion, and the corneal intra-stromal infiltration also increased. After bacterial cultivation in the first encounter, bacteria were inoculated and cultivated in MacConkey agar at an intermediate level in all the specimens (Fig. 2). Achromobacter xylosoxidans was identified using a Vitek 2 GN colorimetirc identification card (Biomerieux Vitek 2 System, Hazelwood, MO, USA) and is known to be sensitive to amikacin, ceftazidime, and tobramycin. Accordingly, the patient was treated with fortified ceftazidime and fortified amikacin with an initial one-hour interval and a late reduced frequency and also with 2% homatropine (Ocuhomapine Eye) and 0.3% hyaluronic acid (Hyalein mini). The symptoms gradually improved three days after treatment, and complete epithelialization of the lesion was observed with corneal opacity on the sixth day after treatment (Fig. 1B). After 7 days, the patient was treated with fortified ceftazidime and fortified amikacin and 0.3% hyaluronic acid four times a day. Her corrected visual acuity in the right eye was 20 / 25.
Case 2
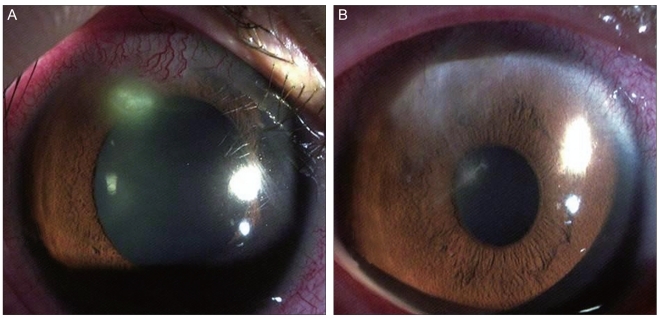
A 21-year-old female patient was transferred to our hospital due to right ocular pain and reduced vision. She had been wearing soft contact lenses for five years. She had no medical history of ocular injury, surgery, or treatment with ophthalmic or systemic steroids. No abnormal finding was observed in the blood test. The patient's corrected visual acuity was 20 / 30 in both eyes, and her intraocular pressure was 15 mmHg in the right eye. Her right bulbar conjunctiva near the lesion had hyperemia, and a 0.5 × 0.5 mm corneal epithelial defect was seen with interstitial infiltration and peripheral neovascularization toward the 7 o'clock direction (Fig. 3A). There were no abnormal findings in the lens, vitreous body, or fundus. A bacteriological test was conducted on her contact lenses, lens cases, and lens washing agent. Her right ocular bacterial ulcer was treated with gatifloxacin (Gatiflo), with an initial one-hour interval and a late reduced frequency; with 2% homatropine (Ocuhomapine Eye) three times a day, and 0.3% unpreserved hyaluronic acid (Hyalein mini) eight times a day. Culture results revealed the presence of Achromobacter xylosoxidans. Therefore, we added fortified tobramycin with an initial one-hour interval and a late reduced dose four times a day to the previous eye drop prescription. Complete epithelialization of the lesion was observed with corneal opacity on the sixth day after treatment (Fig. 3B). The patient's corrected right ocular visual acuity was 20 / 20.
Discussion
Achromobacter xylosoxidans is an anaerobic, Gram-negative bacillus that has motility due to its peritrichous cilia. In 1971, Yabuuchi and Oyama [6] first reported it in a patient with chronic otitis media. A. xylosoxidans has been known to exist not only in humans as ear and intestinal microbial flora, but also in soil and water. Recently, nosocomial infections from dialysis solutions, saline solutions, and humidifier solutions have been reported. These infections are especially known to cause opportunistic infection in patients with reduced immunity due to the presence of tumors, hematologic disease, organ transplants, hypogammaglobulinemia, AIDS, late renal failure, or cystic fibrosis [7-15].
According to previous studies, Achromobacter xylosoxidans is known to be sensitive to piperacillin, ceftazidime, imipenem, and trimethoprim-sulfamethoxazole; resistant to aminoglycoside, ampicillin, aztreonam, and most cephalosporins; and sensitive/resistant to cefepime or ciprofloxacin. Reddy et al. [16] reported that ceftazidime and amikacin were the best drugs for initial treatment of infection with this organism. In their study, antibiotic susceptibility was 90% to ceftazidime and 70% to amikacin. Since the pathogen is resistant to many antibiotics and has many variations, it is important to select an appropriate antibiotic based on a sensitivity test after the spread and cultivation of the pathogen. Pathogens associated with opportunistic infections in hospitals have recently become isolated in keratitis caused by contact lens use. It is likely that wearing contact lenses causes hypoxia and increased carbon gas, by which lactic acid in the corneal epithelium increases and leads to a reduced rate of lactic acid degradation in the corneal epithelium, an epithelial defect due to glycogen insufficiency in the corneal epithelium, and an increased possibility of pathogen invasion [17,18]. In addition, if biofilm is formed on the surface of the contact lens or its storage case, an appropriate environment for bacterial proliferation results. This could lead to a reduced bactericidal effect upon disinfection and increased resistance to members of the immune system such as antibodies or macrophages. It is also likely that the use of a contaminated saline solution, inappropriate disinfection, or direct contact with contaminated water increases the risk of corneal infection. For example, the use of extended-wear lenses, overnight lenses, poor maintenance of the contact lens case, reusing or topping off contact lens solution, and poor lens hygiene contribute to the contamination of contact lenses. In our case, the patients did not wash their hands before handling the contact lenses. Therefore, we suspect that the hands were contaminated by the pathogen, which then spread to the contact lenses, lens solution, and case through the contaminated hands. If Achromobacter xylosoxidans is suspected as a causative pathogen that causes corneal ulcers, a cultivation examination of the contact lenses and washing agent, in addition to the specimens collected from the lesion, is required. Especially in cases of patients with penetrating keratoplasty, patients to whom steroids have been locally or systemically administered, and patients with low immunity, corneal ulcers caused by Achromobacter xylosoxidans should be considered.
To the best of our knowledge, there are ten reported cases of Achromobacter xylosoxidans keratitis in the related literature. Most of these reports focus on patients whose corneal immune systems became impaired after undergoing keratoplasty or after applying steroid eye drops [19-23]. Recently, Reddy et al. [16] reported on the outcome of treatment of Achromobacter xylosoxidans keratitis in patients with trauma, keratoplasty, and endophthalmitis. In their study, the symptoms of all patients except one were resolved with topical antibiotic therapy. Linke et al. [24] also reported a case of bilateral Achromobacter xylosoxidans keratitis after LASIK.
In the present case, it was suspected that keratitis was induced by contact lens usage. Therefore, microbiological examinations were performed on the corneal scrapings, contact lenses, contact lens cases, and lens-washing agents. The growth of Achromobacter xylosoxidans from all specimens was confirmed, which made it possible to ascertain that the contact lenses were the direct cause of the keratitis.
The occurrence of Achromobacter xylosoxidans keratitis may be underreported in the literature because of the difficulty of positively identifying the organism. Achromobacter xylosoxidans share many morphologic similarities with other Gram-negative rods. Achromobacter xylosoxidans keratitis in healthy people without risk factors is indeed rare. This notwithstanding, the organism should be considered a rare but potential pathogen for lens-induced keratitis in healthy hosts.
Acknowledgements
This study was supported by research fund from Chosun University, 2011.
Notes
No potential conflict of interest relevant to this article was reported.