Anterior Ischemic Optic Neuropathy in a Patient with Churg-Strauss Syndrome
Article information
Abstract
We describe a patient with Churg-Strauss syndrome who developed unilateral anterior ischemic optic neuropathy. A 54-year-old man with a history of bronchial asthma, allergic rhinitis, and sinusitis presented with sudden decreased visual acuity in his right eye that had begun 2 weeks previously. The visual acuity of his right eye was 20 / 50. Ophthalmoscopic examination revealed a diffusely swollen right optic disc and splinter hemorrhages at its margin. Goldmann perimetry showed central scotomas in the right eye and fluorescein angiography showed remarkable hyperfluorescence of the right optic nerve head. Marked peripheral eosinphilia, extravascular eosinophils in a bronchial biopsy specimen, and an increased sedimentation rate supported the diagnosis of Churg-Strauss syndrome. Therapy with methylprednisolone corrected the laboratory abnormalities, improved clinical features, and preserved vision, except for the right central visual field defect. Early recognition of this systemic disease by ophthalmologists may help in preventing severe ocular complications.
Churg-Strauss syndrome (CSS) is a systemic necrotizing vasculitis involving multiple organ systems. Despite multisystem involvement, ocular manifestations have been very rarely reported [1]. The mechanism of optic neuropathy is most probably acute ischemic changes of the anterior optic nerve due to direct involvement of the short posterior ciliary arteries by inflammation of the vessel wall [2]. To the best of our knowledge, the occurrence of anterior ischemic optic neuropathy (AION) in CSS has not been reported in Korea. We believe this to be the first report of AION associated with CSS in Korea.
Case Report
A 54-year-old man presented to our department after referral for decreased visual acuity in his right eye for 2 weeks. The patient was being treated in the pulmonology department for a 6-month history of bronchial asthma and allergic rhinitis with parasinusitis. The visual symptom occurred simultaneously with hemoptysis. Visual acuity was 20 / 50 in the right eye and 20 / 20 in the left. Both pupils reacted normally to light and accommodation. Ocular movements, anterior segments, and intraocular pressure were normal in both eyes. There was no sign of inflammation in the anterior chamber and vitreal cavity.
Funduscopy of the left eye was normal with a cup/disc ratio of 0.4, but revealed pallid right optic disc edema and splinter hemorrhages at the disc margin (Fig. 1A). Goldmann perimetry showed central scotomas in the right eye (Fig. 1B). Fluorescein angiography showed localized filling delay and late hyperfluorescence of the right optic nerve head (Fig. 1C and 1D). In addition, localized filling delay in the peripapillary choroid was demonstrated. There were no abnormalities seen in the left eye.
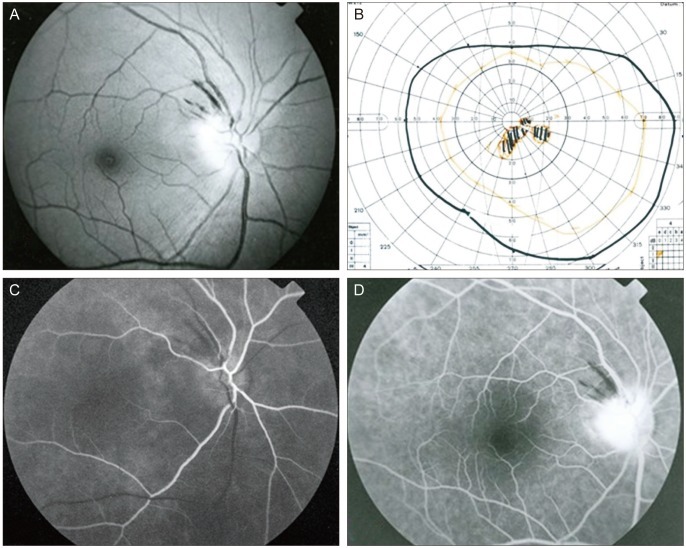
(A) Fundus photograph of the right eye. Diffusely swollen optic disc and splinter hemorrhages along the superotemporal arcade. (B) Goldmann perimetry revealed central scotoma. (C) Fundus fluorescein angiograms of the right eye demonstrate localized filling delays of the optic disc. Optic disc filling delay is an important feature for the diagnosis of anterior ischemic optic neuropathy. (D) Late hyperfluorescence of the right optic nerve and splinter hemorrhages at the disc margin in the right eye are seen.
Laboratory evaluation revealed a hematocrit of 44.9%, and a white blood cell count of 13,600/uL with 34% eosinophilia (normal range, 4,700-9,000/uL with 0.2%-5.4%). The erythrocyte sedimentation rate was 30 mm/hr and the C-reactive protein was 1.72 mg/dL (normal ranges, 0-16 mm/hr and 0-1 mg/dL, respectively). Total IgE was 323.4 IU/mL (normal range, 0-300 IU/mL), anti-nuclear antibody was positive, and the following results were all negative: 2 IgG antineutrophil cytoplasmic antibodies (ANCAs, cytoplasmic-ANCA and perinuclear-ANCA), and anti dsDNA antibodies. A bone marrow biopsy showed 17.2% eosinophils (eosinophilic hyperplasia), suggesting the hypereosinophilic syndrome. Multiple dirty-appearing nodules in the trachea and both main bronchi were found by bronchoscopy and sections of a paraffin-embedded nodule specimen demonstrated diffuse eosinophilic infiltration of the bronchial wall. Systemic evaluation revealed no evidence of hypertension, diabetes, dyslipidemia, cardiovascular disease or any other systemic disease. He was a non-smoker and had no history of intraocular surgery.
These clinical, laboratory, and pathology results established the diagnosis of anterior ischemic optic neuropathy associated with CSS. The patient was treated with 24 mg of methylprednisolone daily. After 1 month of treatment, his visual acuity increased to 20 / 25 in the right eye and his respiratory symptoms were much improved. Follow-up right eye funduscopy revealed that the papilledema had subsided and the splinter hemorrhages at the disc margin were resolved. However, Goldmann perimetry performed 1 month later showed a residual central visual field defect. The peripheral eosinophil count dropped to 4.7%, and the sedimentation rate and the C-reactive protein concentration dropped to normal values.
Discussion
CSS was identified initially by Churg and Strauss as a syndrome characterized by allergic granulomatosis and angiitis. It is a systemic necrotizing vasculitis featuring peripheral eosinophilia, recurrent lung infiltrates, asthma, and peripheral neuropathy [3]. In 1990, the American College of Rheumatology established diagnostic criteria for the diagnosis of CSS, and the presence of 4 of the 6 criteria results in a specificity of 99.7% [4]. These criteria include asthma, eosinophilia >10%, mononeuropathy or polyneuropathy, nonfixed pulmonary infiltrates, paranasal sinus abnormalities, and the presence of extravascular eosinophils on biopsy. The diagnosis of CSS in our patient was based on the presence of eosinophilia (34%), asthma, parasinusitis, and extravascular eosinophilia demonstrated on bronchial biopsy.
CSS is associated with a broad range of ocular conditions, including granulomatous conjunctivitis, retinal vascular occlusion, and cranial neuropathy, including optic neuropathy [2,5-11]. Anterior ischemic optic neuropathy may present as a frequent complication of systemic granulomatous arteritis [12]. Although giant cell arteritis is the most frequently noted and best known systemic granulomatous arteritis, other vasculitis types may also be related to AION [13]. In CSS, AION can cause unilateral or bilateral blindness [2,3,8,9,12-15]. Visual loss in systemic arteritis may present as a consequence of retinal or optic nerve infarction and the arteritic form of AION (A-AION) is associated with a higher degree of visual loss than is noted in the nonarteritic variant [13,14]. Early massive visual loss is strongly suggestive of A-AION, but the presence of perfectly normal visual acuity does not rule out A-AION [16]. In 5 reports involving patients with CSS who developed AION, all of the patients developed visual losses as the result of anterior ischemic optic neuropathy [2,8-11]. Each of the patients presented with sudden visual loss to the level of counting fingers or worse, in association with optic disc swelling. Despite these treatments, one patient experienced a profound bilateral visual loss with bilateral optic nerve involvement [2]. Three patients experienced no change in vision after the initiation of treatment with high-dose corticosteroids and 1 patient experienced an improvement in hand-motion vision to 20 / 60 after treatment with steroids [8-11]. These reports indicate that immunosuppressive therapy may, in some cases, reduce or reverse visual losses in patients with CSS-associated optic neuropathy [4].
The clinical course of the optic disc edema of this patient and the incomplete resolution of his visual field defect are indicative of anterior ischemic optic neuropathy [3]. In our patient, ophthalmoscopic findings and clinical and laboratory results were most consistent with a diagnosis of CSS-associated arteritic AION. The patient's visual improvement may have been the result of early corticosteroid administration prior to additional injury to the optic nerve head [3].
As in our case, other reports have presented evidence to suggest that corticosteroid or immunosuppressive therapy may bring about a prolonged remission [15,17]. These reports emphasize the importance of early diagnosis and intervention for the treatment of this pathologic condition [8]. Therefore, we must echo the recommendations of other researchers that CSS patients should undergo neuro-ophthalmic evaluations and, should abnormal changes of the optic nerve head be detected, early corticosteroid treatment should be administered [3].
Notes
No potential conflict of interest relevant to this article was reported.