Association between Exudative Age-related Macular Degeneration and the G6721T Polymorphism of XRCC7 in Outdoor Subjects
Article information
Abstract
Purpose
To investigate whether the G6721T polymorphism (rs.7003908) of the non-homologous end-joining DNA repair XRCC7 gene contributes to the development of exudative age-related macular degeneration (ARMD).
Methods
The present case-control study consisted of 111 patients with exudative ARMD and 112 sex frequency-matched healthy controls that were randomly selected from unrelated volunteers in the same clinic. Genotypes were determined by the Restriction Fragment Length Polymorphism (PCR-RFLP) based method. Logistic regression was used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for ARMD risk associated with polymorphism of XRCC7. In all analysis the GG genotype was considered to be the reference genotype.
Results
There was no significant association between genotypes of XRCC7 and susceptibility to ARMD. Considering the significant difference in age distribution between cases and controls, age was used as a covariate in further analysis. After ORs were adjusted for age, the same result was observed. In the next step we stratified our subjects into outdoor and indoor groups according to their job titles. The outdoor and indoor patients were occupationally exposed to sunlight and not exposed to sunlight, respectively. Our present study showed that among indoor subjects there was no association between XRCC7 polymorphism and susceptibility to ARMD. However, among outdoor subjects, the GT + TT genotypes compared to the GG genotype increased the risk of ARMD (OR, 3.13; 95% CI, 1.04-9.39; p = 0.042).
Conclusions
Our study revealed that the T allele of the G6721T polymorphism of XRCC7 increased the risk of ARMD among outdoor subjects.
Familial aggregation has been demonstrated for age-related macular degeneration (ARMD) [1-3] and it is likely that both genetic susceptibility [1-4] and environmental risk factors [5-9] play a role. A genome wide scan for ARMD provides evidence for linkage to potential loci on human chromosome 8 [10].
A relationship between long-term sunlight exposure and increased risk of ARMD has been suggested [6] and epidemiologic evidence indicates a trend toward association between severity of light exposure and ARMD [7,8]. Short wavelength radiation and blue light induce significant oxidative stress to the retinal pigment epithelium (RPE). ARMD results from damage to the RPE cells, which is mainly caused by oxidative stress. The stress also affects the DNA of RPE cells, which promotes genome instability in these cells. Therefore, individuals with impaired DNA repair may be more susceptible to ARMD [9].
Gene XRCC7 (MIM: 600899, GenBank accession no: NM_001469) encodes the catalytic subunit of a nuclear DNA-dependent serine/threonine protein kinase (DNA-PK). As the catalytic subunit of the DNA-PK complex, XRCC7 aids in the recognition and repair of DNA double-strand breaks (DSBs) [11]. DNA-PK activity is activated by binding to free DNA ends and it catalyzes the rejoining of DSB [12]. Thus, DNA-PK activity is essential for non-homologous end joining and V (D) J recombination.
Deficiencies in DNA-PK activity are clinically significant. Mice with inactivated components of DNA-PK show severe combined immunodeficiency as well as ionizing radiation hypersensitivity [13,14]. Cells defective in DNA-PK components are hypersensitive to killing by ionizing radiation due to an inability to repair DSBs effectively [15].
The gene encoding XRCC7 is located on human chromosome 8q12 [16]. It has been suggested that the genetic G6721T polymorphism of human XRCC7 (rs.7003908), in intron 8, may regulate splicing and cause mRNA instability [16]. The association between the G6721T polymorphism of XRCC7 and cancers has been studied [17-22]. Additionally, human chromosome 8, where the gene encoding XRCC7 is located, has been associated with an increased risk of ARMD [10]. Since ARMD results from damage to RPE cells caused by oxidative stress and the G6721T polymorphism of XRCC7, may regulate splicing and cause mRNA instability [16], we hypothesize that XRCC7 polymorphism is associated with ARMD. To the best of our knowledge, there is no report on the association between XRCC7 polymorphism and ARMD.
Materials and Methods
Subjects
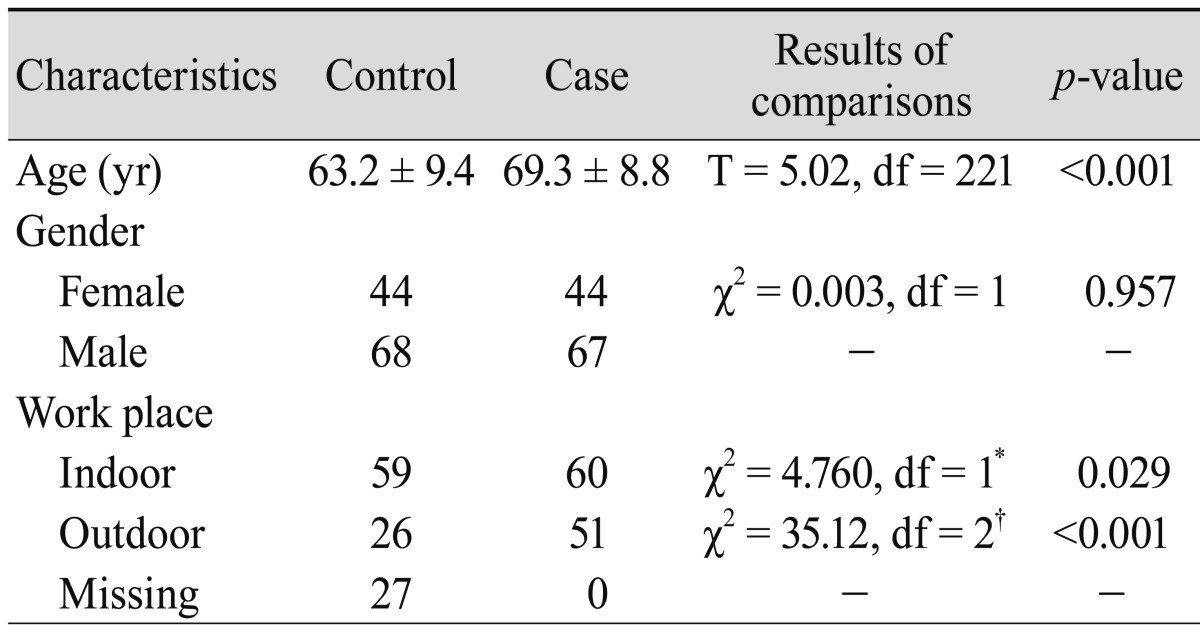
A detailed description of study subjects is located in our previous reports [23,24]. We lost the DNA sample of a patient and therefore, the present hospital-based case-control study consisted of 111 patients (44 females, 67 males) with exudative ARMD. Also, 112 sex frequency-matched individuals (44 females, 68 males) were randomly selected from unrelated volunteers in the same clinic and used as controls. The mean age (SD) of the patients and the controls was 69.3 (8.8) and 63.2 (9.4) years, respectively. There was significant difference in age distributions between patients and controls (t = 5.02, df = 221, p < 0.001). The Iranian population is one of the most heterogeneous populations [25,26]. Therefore, we selected our patients and controls from the same ethnical religious group (Persian Muslims living in Fars province, southern Iran). This study was approved by the local ethics committee and informed consent was obtained from all participants.
The study subjects were divided into two groups: outdoor (farmers, drivers, etc.) and indoor (housewives, teachers, etc.), according to their job titles. The outdoor and indoor patients were occupationally exposed to sunlight and not exposed to sunlight, respectively.
DNA extraction and genotyping analysis
Genomic DNA was extracted from whole blood samples. Genotypic analysis for the XRCC7 polymorphism was determined by Restriction Fragment Length Polymorphism (PCR-RFLP) assay, as described previously [18]. A negative control containing all reagents but water instead of the DNA template was included to each amplification set. To test for contamination, negative controls (tubes containing the PCR mixture, without the DNA template) were incubated in every run. Any sample with an ambiguous result due to low yield was retested and a random selection of 15% of all samples was repeated. No discrepancies were discovered upon replicate testing.
Statistical analysis
A chi-square test was performed for each polymorphism to determine if the control samples demonstrated Hardy-Weinberg equilibrium. Unconditional logistic regression was used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for ARMD risk associated with the genetic polymorphism of XRCC7. In all analysis the GG genotype was used as the reference genotype. Considering the significant age difference between patients and controls, in further analysis, logistic regression was used to calculate ORs and 95% CIs for the various genotypes after adjusting for age.
Data on work place in the control subjects were missed for some participants. In order to also study the potential effect of the work place on ARMD risk as well as the risk associated with genotypes of XRCC7, the "sensitivity analysis" was used [26]. For this analysis we tested two assumptions of the missing data of the work place in the control group: it was outdoor and indoor.
Statistical analysis was performed using the SPSS ver. 11.5 (SPSS Inc., Chicago, IL, USA). A probability of p < 0.05 was considered statistically significant. All statistical tests were two-sided.
Results
The general characteristics of ARMD patients and the control group are summarized in Table 1. The work place significantly differed between cases and controls (p = 0.029). The control and patient groups were initially divided into two sex groups. Since genotypic frequencies showed no statistical differences between sex groups, the sex groups were pooled (for control subjects: χ2 = 0.947, df = 2, p = 0.623; for patient group: χ2 = 1.039, df = 2, p = 0.595).
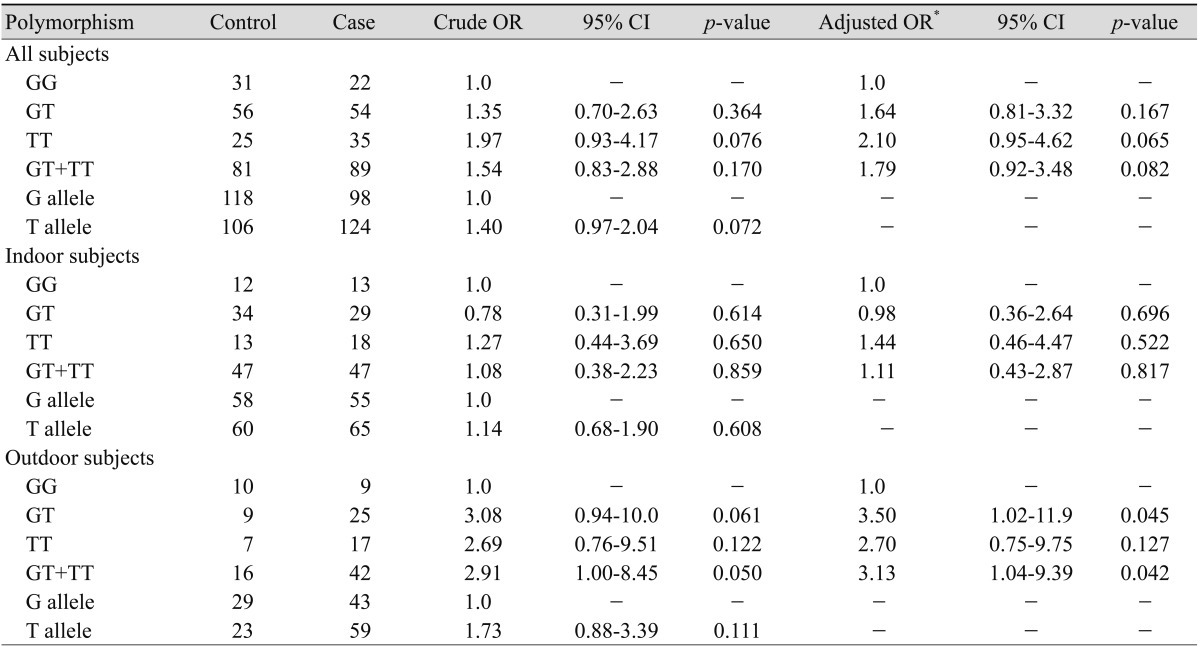
A cross-tabulation by workplace (indoor and outdoor) and XRCC7 genotypes of the cases and controls is presented in Table 2. The prevalence of the T allele of the G6721T polymorphism in our control and case subjects was 47.3% and 55.9%, respectively. The genotypic frequencies of the G6721T polymorphism were consistent with those expected from the Hardy-Weinberg equilibrium (for control subjects: χ2 = 0.001, df = 1, p = 0.976; for patient group: χ2 = 0.020, df = 1, p = 0.887). The frequency of GG, GT, and TT genotypes were 27.7%, 56.0%, and 22.3% among controls and 19.8%, 48.7%, and 31.5% among cases, respectively.
There was no significant association between genotypes of XRCC7 and susceptibility to ARMD (Table 2). Considering the significant age difference between the patients and controls (see Materials and Methods section), age of participants was used as a covariate in further analysis. After ORs adjustment for age, the same results were observed (Table 2).
In the next step we stratified our subjects into two groups according to their job titles: outdoor (farmers, drivers, etc.) and indoor (housewives, teachers, etc), as mentioned in Materials and Methods. Our present study showed that among indoor subjects there was no association between XRCC7 polymorphism and susceptibility to ARMD (Table 2). However, among outdoor subjects a borderline association was observed. The genotypes of GT + TT compared to the GG genotype increased the risk of ARMD (OR, 3.13; 95% CI, 1.04-9.39; p = 0.042).
The results of the present study have some limitations. Data on the work place, which is known to be associated with risk of ARMD, were missing for some participants. Using "sensitive analysis" it is possible to estimate the potential effect of this variable on the study by assuming various degrees of maldistribution of the variable in the control group and seeing how it would affect the results. As mentioned in "Statistical analysis" section, we tested two distributions for the missing data among controls. Statistical analysis showed the same results on association between the XRCC7 polymorphism and risk of ARMD. Assuming all missing data were indoor, the comparisons of GT vs. GG (OR, 1.09; 95% CI, 0.53-2.24; p = 0.800) and TT vs. GG (OR, 1.85; 95% CI, 0.81-4.23; p = 0.142) were not significant. Alternatively, assuming all missing data were outdoor, the comparisons of GT vs. GG (OR, 2.39; 95% CI, 0.90-6.38; p = 0.080) and TT vs. GG (OR, 2.99; 95% CI, 1.01-8.84; p = 0.048) were significantly associated with the risk of ARMD. Therefore the main finding of the present study is that outdoor working subjects with the TT genotype of XRCC7 are at an increased risk for developing ARMD compared to the reference group (GG genotype).
Discussion
The prevalence of the T allele of the XRCC7 G6721T polymorphism in our control subjects (47.3%) was similar to those reported from Caucasian populations [17-22].
Our present finding indicates that there is no significant association between genotypes of XRCC7 and susceptibility to ARMD, before and after adjusting for age of participants (Table 2). It should be noted that epidemiologic evidence indicates a relationship between long-term sunlight exposure and increased risk of both ARMD [6-8] and cataracts [27]. In order to investigate the influence of the XRCC7 polymorphism and work place, we stratified the subjects into two groups according to their job titles: outdoor and indoor subjects. Missing data of the work place of control subjects is an important limitation of the study. Therefore we performed a "sensitivity analysis" as mentioned in the Materials and Methods section. The results of "sensitivity analysis" indicate that outdoor working subjects with the TT genotype are at an increased risk for developing ARMD compared to the reference group (GG genotype). Taken together the association between the XRCC7 G6721T polymorphism and ARMD risk is a true association (see last paragraph of Results section). The present findings suggest that the T allele may be a risk allele, and this XRCC7 polymorphism may be a marker for susceptibility to ARMD in outdoor working patients. It may be suggested that sunlight exposure induces significant oxidative stress to the RPE cells, which promotes genome instability in these cells [6-9]. On the other hand, the G6721T polymorphism of XRCC7 may regulate splicing and cause mRNA instability [17]. Therefore, individuals with the T allele have lower DNA repair capacity, and subsequently may be more susceptible to ARMD if oxidative stress affects their RPE cells.
It is also important to keep the limitations of this study in mind when considering the present findings. The main limitation of the present study is the small sample size and thus the limited statistical power to detect difference(s) between the case and control groups, especially when the participants were stratified into indoor and outdoor categories. Another limitation of our study is the measurement of sunlight exposure as a dichotomous variable (indoor versus outdoor). The difference in subjects' age between cases and controls is another limitation of our study. Our findings should be confirmed by a large-scale study. It is recommended that in future studies, sunlight exposure should be measured as a variable with more categories or as a continuous variable.
Acknowledgements
The authors are indebted to the participants for their close cooperation. This study was supported by Shiraz University. The authors have no conflict of interest in relation to this study.
Notes
No potential conflict of interest relevant to this article was reported.