Short-term Effectiveness of Intravitreal Bevacizumab vs. Ranibizumab Injections for Patients with Polypoidal Choroidal Vasculopathy
Article information
Abstract
Purpose
To compare the effectiveness of intravitreal injections of bevacizumab and ranibizumab in patients with treatment-naive polypoidal choroidal vasculopathy (PCV).
Methods
Records from 106 consecutive patients who received intraviteral bevacizumab (n = 58, 1.25 mg) or ranibizumab (n = 52, 0.5 mg) for treatment of PCV were retrospectively reviewed. After three initial monthly loading injections, injection was performed as needed. The main outcome measures included best-corrected visual acuity (BCVA), foveal central thickness (FCT) as assessed by spectral domain optical coherence tomography, and the changes in polypoidal lesions based on an indocyanine green angiography.
Results
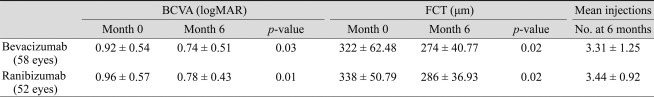
The average number of injections was 3.31 ± 1.25 in the bevacizumab group and 3.44 ± 0.92 in the ranibizumab group. Mean logarithm of the minimum angle of resolution of BCVA from baseline to 6 months after injection improved by 0.17 in the bevacizumab group (p = 0.03) and by 0.19 in the ranibizumab group (p = 0.01). Average FCT decreased from 322 ± 62.48 µm to 274 ± 40.77 µm in the bevacizumab group (p = 0.02) and from 338 ± 50.79 µm to 286 ± 36.93 µm in the ranibizumab group (p = 0.02). Polyp regression rate was 20.7% (12 of 58 eyes) in the bevacizumab group and 21.2% (11 of 52 eyes) in the ranibizumab group. There was no statistically significant difference between groups in BCVA improvement achieved, FCT improvement achieved, and polyp regression rate between groups.
Conclusions
Intravitreal injections of bevacizumab and ranibizumab have similar effects in stabilizing of visual acuity, macular edema, and regression of polypoidal complex in PCV eyes over the short term.
Polypoidal choroidal vasculopathy (PCV) is characterized by a branching vascular network with polypoidal shaped choroidal vascular lesions that result in subretinal leakage, subretinal hemorrhage, and pigment epithelial detachment [1-4]. When pathologic changes associated with PCV extend to the subfoveal area, visual prognosis may be poor. Sho et al. [3] have found severe vision loss caused by persistent serous detachment, atrophy of retinal pigment epithelium (RPE), and submacular hemorrhage in 34.5% (38 of 110 eyes) of PCV patients.
There is no widely accepted and effective method of treatment for PCV. Photodynamic therapy (PDT) with verteporfin or intravitreal injection of anti-vascular endothelial growth factor (VEGF) has recently been administered for treatment of PCV eyes, and encouraging results have been reported [5-10].
The pathogenesis of PCV is not fully understood. However, VEGF may have a role in pathogenesis. Compared with normal controls, VEGF concentrations in the aqueous were found to be markedly increased in PCV eyes [11], and there is a strong expression of VEGF in PRE cells of PCV specimens [12]. These reports support the use of anti-VEGF treatment in PCV eyes.
Ranibizumab is a humanized, anti-VEGF antibody that inhibits all forms of biologically active VEGF-A [13]. Treatment with ranibizumab appears to significantly decrease bleeding and exudation in PCV [10]. Bevacizumab (a humanized, full-length anti-VEGF antibody) also appears to have a treatment effect in PCV eyes [8,9]. Kokame et al. [12] reported that continuous, monthly intravitreal ranibizumab injections were well tolerated in PCV patients. Additionally, patients also showed stabilized vision and decreased macular edema.
Differences in the therapeutic effect between bevacizumab and ranibizumab in neovascular age-related macular degeneration are controversial. A large head-to-head clinical trial sponsored by the National Eye Institute for evaluation of the efficacy of ranibizumab and bevacizumab is ongoing and a more definite result is anticipated in the future [13]. However, to the best of our knowledge, there have been no reports about the differences between bevacizumab and ranibizumab for treatment of PCV. The purpose of this current study is to determine whether or not there are differences in short-term effectiveness between bevacizumab and ranibizumab for treatment of PCV.
Materials and Methods
After obtaining approval by the institutional review board, a retrospective medical record review was conducted using records from 106 consecutive patients (110 eyes) who were treated with intravitreal anti-VEGF agents for PCV at Kim's Eye Hospital, Konyang University College of Medicine from November 2008 to June 2010.
Patients were included if they met all of the following criteria: 1) confirmation of PCV with fluorescein angiography (FA) and indocyanine green angiography (ICGA) performed using a confocal laser scanning system (HRA-2; Heidelberg Engineering, Dossenheim, Germany) at the first visit. We only included patients whose ICGA showed a branching vascular network with polypoidal shaped choroidal vascular lesions, 2) patients who were treated with only one type of anti-VEGF agent (either bevacizumab or ranibizumab), and 3) a minimum follow-up period of 6 months.
Exclusion criteria were the following: 1) combination therapy of more than one anti-VEGF agent, 2) prior treatment with PDT, 3) pathologic myopia, 4) idiopathic choroidal neovascularization (CNV), 5) other secondary CNV, 6) other ocular disease that could affect visual acuity, 7) trauma during the study or in the fellow eye, 8) aphakia, or 9) previous vitreoretinal surgery.
Treatment and re-treatment protocols were the same for both groups of patients. Evidence of PCV with recent visual deterioration was an indication for treatment. We performed three consecutive, monthly-loading dose injections in every patient. After the initial loading injections at the time of diagnosis, retreatment for each patient was planned as a 'retreat as needed' protocol. Anti-VEGF agents were re-injected on a monthly basis if objective visual deterioration of more than two lines, persistent exudates and hemorrhage, or evidence of an active PCV lesion were observed on FA, ICGA, or optical coherence tomography (OCT), were observed. Best-corrected visual acuity (BCVA) was obtained every one or two months using the Snellen chart. Patients also underwent an ophthalmic examination, including a slit-lamp evaluation and fundus examination, as well as OCT (Spectral OCT/SLO; OTI Ophthalmic Technologies Inc., Miami, FL, USA), FA, ICGA, or a combination thereof.
Foveal center thickness (FCT) was assessed by OCT using six diagonal fast and slow 6-mm scans. Retinal thickness of the 1-mm central retina was obtained by a fast macular scan. Only well-centered scans without overt algorithm failure messages were selected for analyses.
Intravitreal antivascular endothelial growth factor injection
The off-label nature of the treatment and its potential risks and benefits were discussed in detail with all patients, and signed informed consent was obtained from all patients. Patients received 1.25 mg of bevacizumab or 0.5 mg of ranibizumab. Prior to administration of the injection, topical anesthesia was applied, and 10% povidone-iodine was used for scrubbing of eyelid and lashes. Following the application of povidone-iodine eyedrops (1.25%), a sterile lid speculum was placed into place. Intravitreal injection was performed with a 30-gauge needle at 3.5 to 4 mm from the inferotemporal limbus. Pressure was applied to the injection site using a sterile cotton swab, for 1 minute. All patients were instructed to apply antibiotic eye drops for one week.
Statistical analysis
SAS (SAS Institute Inc., Cary, NC, USA) was used for all analyses. Frequencies were compared between treatment groups using chi-square tests. The changes in BCVA and FCT between baseline and the 6 month follow-up were analyzed with a 1-tailed, paired t-test. For continuous variables, medians for baseline, final values, change, and percent change were compared between bevacizumab and ranibizumab treatment groups using a t-test or a Wilcoxon rank sum tests. A p-value of less than 0.05 was considered significant.
Results
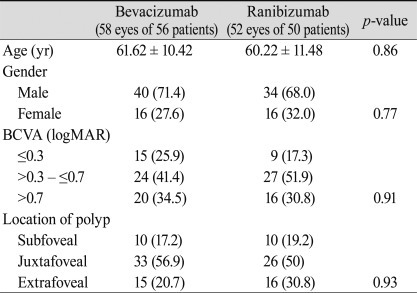
Patient demographics and comparisons of data at baseline are summarized in Table 1. Bevacizumab-treated and ranibizumab-treated patients had similar baseline characteristics for age, sex, distribution of baseline BCVA, location of polyps, or FCT (Table 1). All patients were Korean, and no systemic adverse events were recorded for any of the patients treated with intravitreal injection. No complications, including issues such as endophthalmitis, traumatic lens injury, or retinal detachment, were associated with intravitreal injections.
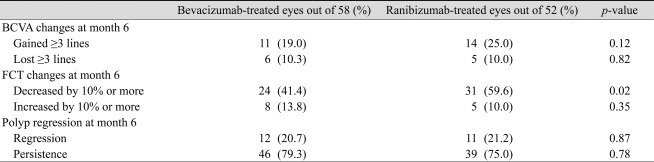
The average number of injections was 3.31 ± 1.25 in the bevacizumab group and 3.44 ± 0.92 in the ranibizumab group. At baseline, the mean BCVA (±standard deviation, SD) in the bevacizumab and ranibizumab groups was 0.92 (±0.54; Snellen equivalent, 20 / 166) and 0.96 (±0.57; Snellen equivalent, 20 / 182), respectively. Six months after treatment, both bevacizumab and ranibizumab groups had significantly increased BCVA to 0.74 (±0.51; Snellen equivalent, 20 / 110; p = 0.03) and 0.78 (±0.43; Snellen equiva lent, 20 / 120; p = 0.01) respectively (Table 2, Figs. 1 and 2). There was no statistically significant difference in BCVA improvement achieved between these two groups (p = 0.83). Six (10.3%) eyes out of 58 eyes in the bevacizumab group and 5 (10.0%) eyes (10.0%) out of 52 eyes in the ranibizumab group showed a loss of ≥3 lines of visual acuity. In either group, no significant difference in proportion of more than 3 lines of visual acuity loss was observed (p = 0.82). There was also no significant difference in proportion of more than 3 lines of visual acuity gain in either group (p = 0.12) (Table 3).
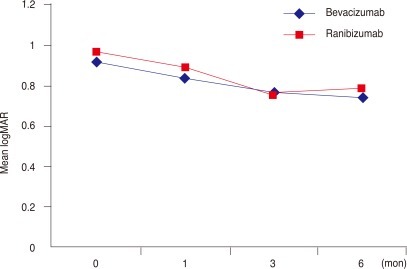
Intravitreal bevacizumab and ranibizumab for polypoidal choroidal vasculopathy: graph showing serial changes in the mean logarithm of the minimum angle of resolution (logMAR) visual acuity from baseline to month 6 post-treatment. The differences in time course between the two groups were not significant. There was a significant decrease in logMAR in both groups.

Intravitreal bevacizumab and ranibizumab for polypoidal choroidal vasculopathy: graph showing serial changes in optical coherence tomography and mean foveal center thickness (FCT) from baseline to month 6 post-treatment. The differences in time course between the 2 subgroups were not significant. There was a significant decrease in FCT in both groups.
Bevacizumab and ranibizumab for PCV: visual acuity, optical coherence tomography, and indocyanine green angiography changes at month 6 after treatment
Mean (±SD) FCT at baseline in the bevacizumab and ranibizumab groups was 322 (±62.48) µm and 338 (±50.79) µm, respectively. Six months after treatment, FCT of both the bevacizumab and ranibizumab groups were significantly decreased to 274 (±40.77) µm (p = 0.02) and 286 (±36.93) µm (p = 0.02), respectively. There was no statistically significant difference in reduction of FCT in either group (p = 0.74) (Table 2). Twenty four of 58 eyes (41.4%) in the bevacizumab-treated group showed a decrease of more than 10% from baseline FCT. Thirty one eyes out of 52 eyes (59.6%) in the ranibizumab-treated group showed a decrease of more than 10% from baseline FCT. No significant difference in the proportion of decrease greater than 10% from baseline FCT was observed in either group (p = 0.35). However, a greater number for ≥10% decreased FCT was observed in the ranibizumab group, and the difference was statistically significant (p = 0.02) (Table 3).
Polyp regression was found in 12 of 58 eyes (20.7%) in the bevacizumab group and in 13 of 52 eyes (25.0%) in the ranibizumab group. No significant difference in polyp regression rate was observed between groups (p = 0.87) (Table 3).
Discussion
PCV is increasingly recognized as a major cause of vision loss throughout the world; however, the incidence of PCV is especially high in Asian countries and in Asian people throughout the world [14,15]. The most important causes of severe visual loss include repeated subretinal hemorrhage and leakage from PCV lesions [3], repeated injuries resulting in atrophy of RPE, or scar change. Although the pathophysiology of PCV is poorly understood, resolving subretinal hemorrhage and decreasing leakage from PCV lesions may be an important and reasonable approach to stabilize visual acuity. Additionally, VEGF concentrations in the aqueous have been found to be markedly increased in PCV eyes [11], and a strong expression of VEGF was observed in PRE cells of PCV specimens [12]. Collectively, evidence appears to support the success of anti-VEGF treatment for PCV eyes.
Ranibizumab, which is specifically for intraocular use, has several theoretical advantages over bevacizumab. Ranibizumab is a humanized anti-VEGF antibody that inhibits all forms of biologically active VEGF-A [16]; treatment with ranibizumab appears to significantly decrease bleeding and exudation in PCV [10,12]. Bevacizumab (a humanized full length anti-VEGF antibody) also appears to have a treatment effect in PCV eyes [8,9]. Considering the molecular weight of each medication (ranibizumab is a 48-kDa Fab fragment, whereas bevacizumab is a complete 149-kDa antibody), ranibizumab may be more effective in treatment of PCV because of its smaller molecular weight and possible deeper penetration to choroidal vascular abnormality lesions in those with PCV [15]. Moreover, ranibizumab is affinity-matured and may provide better VEGF inhibition through stronger molecular binding, compared with bevacizumab. In comparison, penetration of the neural retina up to the choriocapillaries by intravitreal bevacizumab has been demonstrated [17]. And the larger molecular weight of bevacizumab might result in a longer duration of action. Therefore, it may be possible that the clinical efficacies of these two drugs might differ [18].
In the current study, both drugs significantly improved visual acuity in the short term, and showed similar increases in their mean visual acuity from baseline. However, despite the lack of power to determine small changes in visual acuity between bevacizumab and ranibizumab, our results revealed a trend toward a greater number for ≥3-line improvements in the ranibizumab group. Although it is uncertain what factors made this difference in visual acuity between the two groups, these results showing a significantly greater number for ≥10% decreased CFT in the ranibizumab group could have an association.
Macular edema based on FCT significantly improved in both groups and showed similar decreases in mean FCT from baseline. A similar decrease in macular edema was noted after three monthly bevacizumab injections in a study by Lai et al. [9]. Additionally, Kokame et al. [12] reported improvement in macular edema after 6 monthly ranibizumab injections. Findings from these previous reports as well as those of our study collectively suggest that short-term continuous anti-VEGF therapy doses do have a significant effect in reducing macular edema but not in all PCV eyes. In this study, our results revealed a significantly greater number of patients who showed ≥10% decrease in FCT in the ranibizumab group compared to that in the bevacizumab group. It may be that the difference in changes of FCT between the two groups resulted from differences in penetration ability; ranibizumab may have been more anatomically effective than bevacizumab in reducing macular edema with PCV lesions. Further, these results imply that there is a distinct difference in short-term biologic activity between bevacizumab and ranibizumab.
The choroidal vascular branching network and polypoidal complexes have been resistant and poorly responsive to anti-VEGF therapy with bevacizumab or ranibizumab [8,12]. In the Gomi et al. [8] and the Gomi et al. [15] studies, choroidal vascular abnormalities remained in ten of 11 eyes after one to three intermittent injections of bevacizumab. Kokame et al. [12] found that polypoidal complex decreased in four of 12 eyes (33%) after 6 continuous monthly ranibizumab injections. In the current study, polypoidal lesions appeared to be resistant to both anti-VEGF agent, and the polypoidal complex showed a decrease in only 12 of 58 eyes (20.7%) in the bevacizumab group and 13 of 52 eyes (25.0%) in the ranibizumab group. Even though ranibizumab has a theoretically better ability to penetrate through the retina and RPE to the choroidal vascular abnormalities of PCV [16,18], there was no significant difference in polypoidal complex regression between the two groups. The location of the PCV vessels beneath the RPE may prevent sufficient penetration of anti-VEGF drugs to induce PCV regression. This result suggests that PCV may be a different inner choroidal vascular abnormality [19,20] and not just a variant of choroidal neovascularizatoin (CNV).
In recent studies, PDT has shown good results in reduction of leakage and regression of polyps in PCV eyes [5,6]. Particularly, in the EVEREST study, the first randomized and prospective study of PCV treatment, a PDT combination of ranibizumab and PDT monotherapy was effective in completely regressing polyps at month 6 than was ranibizumab monotherapy. However, there was no significant difference in improvement of visual acuity from baseline between PDT combination with the ranibizumab group and the ranibizumab monotherapy group. In addition, severe visual loss due to extensive subretinal hemorrhage is not uncommon after PDT [21], and PDT itself can result in a temporary increase in VEGF [14].
In terms of visual outcome, despite weakness in polyp regression, anti-VEGF monotherapy could be considered for PCV in cases with minimal polyp lesions or in cases with only a branching vascular network. We await long-term results of the EVEREST trial, which can confirm which modality is superior for treatment of PCV. More clinical and basic science studies are necessary to clarify the pathogenesis of PCV and to provide therapeutic guidelines.
Because of the retrospective nature of the study the inherent bias that exists in this study. And because the treatment choice was left to the discretion of the patient and treating physician, some potential for bias does exist. However, in our institute, the preferred PCV treatment with anti-VEGF (except for PDT) shifted from bevacizumab to ranibizumab from 2008 to 2009. Almost all patients were treated with bevacizumab from 2008 to the first half of 2009 and with ranibizumab from the second half of 2009 to date. As a result, this study could be more comparative. Moreover, the similarity in baseline characteristics between the two groups suggests that the groups were well balanced. Another limitation in this study was the absence of a strict protocol for measuring visual acuity, which led to some of the variances in visual acuity that were noted in the two groups and may limit interpretation of these visual acuity results. However, we were able to significantly identify trends in visual improvement after anti-VEGF injection for PCV eyes. A planned randomized, controlled study would be necessary for a more precise determination of the differences between these two treatments.
In summary, bevacizumab and ranibizumab have similar effects on stabilization of visual acuity and macular edema with PCV eyes in the short term. However, ranibizumab appears to be superior to bevacizumab with regard to short-term ability to decrease exudation. Additionally, there is a trend suggesting that ranibizumab may also provide superior visual acuity. Although this study could not provide definite proof that there were significant differences in the visual acuity as result of PCV eyes treated with bevacizumab or ranibizumab, these results appear to demonstrate a possible difference in the biologic activities of the two anti-VEGF agents. These results should be considered clinically when performing combination therapy with PDT or when deciding on a course of anti-VEGF agent for treatment of PCV eyes.
Notes
This paper was presented as a poster at the 104th annual meeting of the Korean Ophthalmological Society in November 2010.
No potential conflict of interest relevant to this article was reported.