Postoperative Astigmatic Outcomes Based on the Haptic Axis of Intraocular Lenses Inserted in Cataract Surgery
Article information
Abstract
Purpose
This study was conducted to compare post-operative astigmatic outcomes of two groups, with-the-rule (WTR) and against-the-rule (ATR) astigmatism patients, according to the haptic axis of intraocular lenses (IOLs) inserted in cataract surgery.
Methods
Seventy-two eyes with WTR astigmatism and 79 eyes with ATR astigmatism had cataract surgery through a clear corneal temporal incision. These two groups of eyes were then each divided into 2 groups based on whether the haptic axis of the inserted IOL was at 180° or 90°. For ATR patients, the outcomes were analyzed according to the three types of IOLs.
Results
There was no difference in corneal astigmatism, but WTR patients with a 180° haptic axis of the inserted IOL and ATR patients with a 90° hepatic axis of the inserted IOL had a significant decrease in postoperative refractive astigmatism (p < 0.05). The changes in ATR astigmatism according to the IOL type were more effective in single-piece acrylic IOLs than in the three-piece polymethylmethacrylate haptic IOL group.
Conclusions
Insertion of the IOL at the 180° haptic axis in WTR patients and at 90° in ATR patients during cataract surgery may have an effect in reducing pre-existing astigmatism. This observed effect was not consistent among the different types of IOLs.
Astigmatism following cataract surgery is of concern to ophthalmologists. There have been reports that 15% to 29% of patients undergoing cataract surgery have more than 1.5 diopters (D) of pre-existing astigmatism [1]. Reducing this pre-existing astigmatism may further improve the visual outcome of cataract surgery. To reduce or eliminate pre-existing corneal astigmatism, many techniques have been attempted such as changing the size of the incision, location, and architecture; changing the suture technique, suture material, and tension; and adding corneal relaxing incisions, and limbal or peripheral relaxing incisions [2-7].
Astigmatism, although primarily corneal in origin, may be produced when any element of the eye, including the fovea, is not centered along the effective optic axis of the eye. Total astigmatism is a vectorial sum of corneal astigmatism and ocular residual astigmatism [8,9]. The anterior corneal surface is the main refracting element of the human eye, contributing to over two-thirds of the eye's total refractive power [10]. Thus, correcting the pre-existing corneal astigmatism through the application of a cataract incision on or close to the steep corneal meridian may result in effective postoperative visual outcomes. Toric intraocular lenses (IOLs) were recently developed to reduce pre-existing astigmatism of cataract patients, regardless of their corneal astigmatism [11,12]. When inserting newly developed aspheric and multifocal IOLs, it is important to consider these components of astigmatism [13-17]. Considering only the indication criteria of astigmatism, which is less than 1 D of corneal astigmatism, may result in unpredicted and unsatisfactory outcomes after inserting these new IOLs in cases of highly astigmatic patients.
Regarding ocular residual astigmatism, the refractive changes induced by IOL tilt and longitudinal displacement are well known. Decentration of the IOL can be produced by rotation (tilt) of the lens implant along its optic axis and/or translation (displacement) of the IOL perpendicular to its optic axis. Similarly, it is possible that IOL positioning in the capsular bag could affect postoperative astigmatism in cataract surgery. Therefore, we evaluated the post-operative astigmatic outcomes of two groups, with-the-rule (WTR) and against-the-rule (ATR) astigmatism patients, according to the haptic axis of the IOLs in cataract surgery.
Materials and Methods
This prospective study consisted of 151 eyes of 138 patients who had cataract surgery between March 2007 and February 2008. Data was obtained from all participants in this study. The Medical Ethics Committee of St. Mary's Hospital, Catholic University of Korea approved the study protocol, and all participants gave informed consent according to the Declaration of Helsinki. The WTR astigmatism group consisted of 72 eyes of 72 patients, and the ATR astigmatism group consisted of 79 eyes of 66 patients. WTR astigmatism was defined as the flattest corneal curvature at 180 ± 20° and ATR astigmatism was defined as the flattest corneal curvature at 90 ± 20° by topography and refraction. One month after the cataract surgery, patients out of the 180 ± 20° range for WTR astigmatism and patients out of the 90 ± 20° range for ATR astigmatism were eliminated from the study.
The pre-operative evaluation included visual acuity, applanation tonometry, refractive error, slit lamp examination, fundus examination, biomicroscopy, keratometry, specular microscopy, and topography (Orbscan II; Orbtek Inc., Salt Lake City, UT, USA). The change in corneal and refractive astigmatism was evaluated by corneal topography and retinoscopy. To correct the difference of the astigmatism axis before and after cataract surgery, the polar value concept described by Naeser et al. [18] was used. The astigmatism correction rate was calculated as follows: astigmatism correction rate = (difference in astigmatism between the preoperative and postoperative astigmatic polar value [AKP] / preoperative AKP) × 100%. All patients had grade 2 cataracts based on the Lens Opacities Classification System III grading system. This information was important in order to minimize error in refraction.
All cataract surgeries were performed by one surgeon using the same technique. A 3 mm clear corneal temporal incision was first made 1 mm from the limbus with a diamond blade. After injecting viscoelastics into the anterior chamber, a continuous curvilinear capsulorrhexis was made slightly smaller then the optic size of the IOL. Hydrodissection and hydrodelineation was done with a balanced salt solution (BSS; Alcon, Fort Worth, TX, USA). Phacoemulsification of the lens nucleus was performed with a phacoemulsifier (Infiniti, Alcon) and the lens cortex was removed by an irrigation/aspiration device. The capsular bag was expanded with viscoelastics and without enlarging the incision, one of three types of IOLs was inserted into the bag: AcrySof SA60AT (Alcon), Rayner C-flex (Rayner, Sussex, UK), and Tecnis ZA9003 (AMO, Santa Ana, CA, USA). After placing the IOL in the bag, IOL rotation was performed to place the haptic axis at 90° or 180° (Fig. 1). The axis of the inserted IOL was randomly selected. The viscoelastics of the anterior chamber were removed by an irrigation/aspiration device and Miochol (Novartis, Basel, Switzerland) was injected into the chamber. Incision sutures were not performed for all patients. None of the patients had complications during or after the surgery.

Scheme of the axis of the inserted intraocular lens (IOL) in the patient's right eye. (A) An imaginary line of the haptic-optic-haptic laying horizontally is defined as the insertion of the IOL at the 180° haptic axis. (B) The vertical line is defined as the insertion of the IOL at the 90° haptic axis.
Patients were divided into four groups according to the pre-operative astigmatism and the haptic axis of the inserted IOL: group 1, WTR astigmatic patients with insertion of the IOL at the 180° haptic axis; group 2, WTR astigmatic patients with insertion of the IOL at the 90° haptic axis; group 3, ATR astigmatic patients with insertion of the IOL at the 180° haptic axis; and group 4, ATR astigmatic patients with insertion of the IOL at the 90° haptic axis. Evaluations were performed 1 day, 1 week, 1 month, and 2 months after the operation and included visual acuity, refractive error, and topography. Only patients with pre-operative and post-operative astigmatism within the range of our definition of WTR and ATR astigmatism by topography and refraction were included in the analysis. Pupil dilatation was done 1 month post-operatively and patients seen with IOL rotation from the original axis of the inserted IOL (±10°) were excluded from the study. A randomized prospective study was done and statistical analysis was performed with SPSS (SPSS Inc., Chicago, IL, USA), using the paired t-test and one-way ANOVA.
Results
WTR patients with insertion of the IOL at the 180° haptic axis (group 1) included 38 eyes, 34 eyes were from WTR patients with insertion of the IOL at the 90° haptic axis (group 2), 36 eyes were from ATR patients with insertion of the IOL at the 180° haptic axis (group 3), and 43 eyes were from ATR patients with insertion of the IOL at the 90° haptic axis (group 4). There were no differences in age or gender between the four groups of patients. The pre-operative mean refractive astigmatism of the 4 groups was 1.32 ± 1.17 D, 1.46 ± 1.09 D, 1.23 ± 0.85 D, and 1.34 ± 0.76 D, respectively. The pre-operative mean corneal astigmatism was 1.20 ± 1.14 D, 1.30 ± 0.79 D, 1.41 ± 0.48 D, and 1.26 ± 1.09 D, respectively, showing no significant difference between the 4 groups.
Outcomes of the with-the-rule patients
The mean refractive astigmatism in group 1 (WTR astigmatic patients with insertion of the IOL at the 180° haptic axis) was 1.00 ± 0.73 D, 1.01 ± 0.77 D, and 1.01 ± 0.81 D 1 week, 1 month, and 2 months post-operatively, respectively. These values were significantly lower than the pre-operative value (1.32 ± 1.17 D; p = 0.037, 0.042, and 0.044, respectively, statistically significant values are indicated as * in the figure). However, the mean refractive astigmatism in group 2 (WTR astigmatic patients with insertion of the IOL at the 90° haptic axis) was 1.34 ± 1.09 D, 1.49 ± 1.11 D, and 1.59 ± 1.30 D 1 week, 1 month, and 2 months after surgery, respectively. These values were not significantly different compared to the pre-operative value (1.46 ± 1.09 D; p = 0.786, 0.793, and 0.812, respectively).
Comparing these two groups, refractive astigmatism was significantly lower in group 1 than group 2 1 week, 1 month, and 2 months after the operation (p = 0.051, 0.050, and 0.048, respectively). The mean corneal astigmatism in the 2 groups showed a slight increase. In group 2, a statistically significant increase of corneal astigmatism 1 week, 1 month, and 2 months after the operation was observed (statistically significant values are indicated as * in the figure). However, there were no significant differences in post-operative corneal astigmatism between the 2 groups, except at 2 months post-operatively (p = 0.321, 0.129, 0.585, and 0.050, respectively) (Fig. 2A and 2B).

The changes in mean astigmatism by intraocular lens (IOL) axis insertion. In the with-the-rule (WTR) astigmatism group by refraction (A), topography measurement (B), and in the against-the-rule (ATR) astigmatism group by refraction measurement (C), and topography measurement (D) (*p <0.05 indicates p-value compared with the preoperative values).
D=diopters.
The changes of the refractive cylinder value 2 months post-operatively were a 0.31 ± 0.36 D decrease in group 1 and 0.07 ± 0.21 D increase in group 2. The changes of the corneal astigmatism value in Topography 2 months after the operation were a 0.06 ± 0.17 D increase in group 1 and 0.35 ± 0.18 D increase in group 2.
Outcomes of the against-the-rule patients
The mean refractive astigmatism in group 4 (ATR astigmatic patients with insertion of the IOL at the 90° haptic axis) was 1.18 ± 0.63 D, 0.97 ± 0.56 D, and 0.91 ± 0.53 D 1 week, 1 month, and 2 months post-operatively, respectively. One and 2 months after the operation, these values were significantly lower than the pre-operative value (1.34 ± 0.76 D; p = 0.049, 0.042, statistically significant values are indicated as * in the figure). The mean refractive astigmatism in group 3 (WTR astigmatic patients with insertion of the IOL at the 180° haptic axis) was 1.20 ± 0.51 D, 1.21 ± 0.56 D, and 1.22 ± 0.40 D 1 week, 1 month, and 2 months post-operatively, respectively. These values showed no significant difference compared to the pre-operative value (1.23 ± 0.85 D; p = 0.573, 0.672, and 0.722, respectively).
Comparing these two groups, the refractive astigmatism as significantly lower in group 4 than group 3, 1 and 2 months post-operatively (p = 0.046, 0.034). However, post-operative corneal astigmatisms in the 2 groups showed a gradual decrease compared to their pre-operative value. There was no significant difference in corneal astigmatism between the 2 groups (p = 0.310, 0.291, 0.170, and 0.302, respectively) (Fig. 2C and 2D).
The changes in the refractive cylinder value 2 months post-operatively were a 0.01 ± 0.45 D decrease in group 3 and 0.43 ± 0.23 D decrease in group 4. The changes in the corneal astigmatism value in topography 2 months post-operatively were a 0.22 ± 0.38 D decrease in group 3 and 0.20 ± 0.55 D decrease in group 4.
Astigmatic polar value
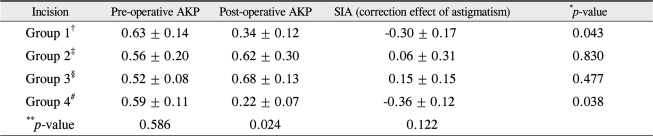
Pre-operative and post-operative refractive astigmatism considering the axis and surgically-induced astigmatism (SIA) were calculated using polar value analysis. Pre-operative AKPs were not different between all the groups, but post-operative AKPs were significantly different. The change of AKP, which is SIA, showed a 0.30 ± 0.17 D decrease of refractive astigmatism in group 1 and a 0.36 ± 0.12 D decrease in group 4. This was statistically significant in groups 1 and 4. Although it showed no statistical significance in groups 2 and 3, the SIA slightly increased, which is consistent with the previous results (Table 1).
Outcomes of the different types of IOLs in ATR astigmatism patients
Patients with ATR astigmatism were divided into 3 sub-groups based on their IOL type. The first sub-group consisted of 27 eyes with a single-piece hydrophobic acrylic IOL (Alcon acrySof SA60AT; optic diameter, 6.0 mm; overall length, 13.0 mm). The second sub-group consisted of 20 eyes with a single-piece hydrophilic acrylic IOL (Rayner C-flex; optic diameter, 5.75 mm; overall length, 12.0 mm). The last sub-group consisted of 22 eyes with a three-piece hydrophobic acrylic optic and a polymethylmethacrylate (PMMA) haptic IOL (AMO Tecnis ZA9003; optic diameter, 5.75 mm; overall length, 12.0 mm).
In the first sub-group (single-piece hydrophobic acrylic IOL), there was no significant difference in corneal astigmatism between insertion of the IOL at the 180° or 90° haptic axis. Refractive astigmatisms of those with IOLs inserted at the 180° haptic axis were 1.15 ± 0.84 D, 0.92 ± 0.48 D, and 0.98 ± 0.54 D 1 week, 1 month, and 2 months post-operatively, respectively. The corresponding post-operative refractive astigmatisms of those with IOLs inserted at the 90° haptic axis were 0.65 ± 1.10 D, 0.53 ± 0.83 D, and 0.59 ± 0.47 D, respectively. There was a significant difference 1 week, 1 month, and 2 months post-operatively between insertion of the IOL at the 180° or 90° haptic axis (p = 0.031, 0.047, and 0.045, respectively). Compared with pre-operative astigmatism values, there were no changes in corneal astigmatism post-operatively (Fig. 3B), but there was a significant decrease in refractive astigmatism 1 week, 1 month, and 2 months after the operation (Fig. 3A, indicated as * in the figure).
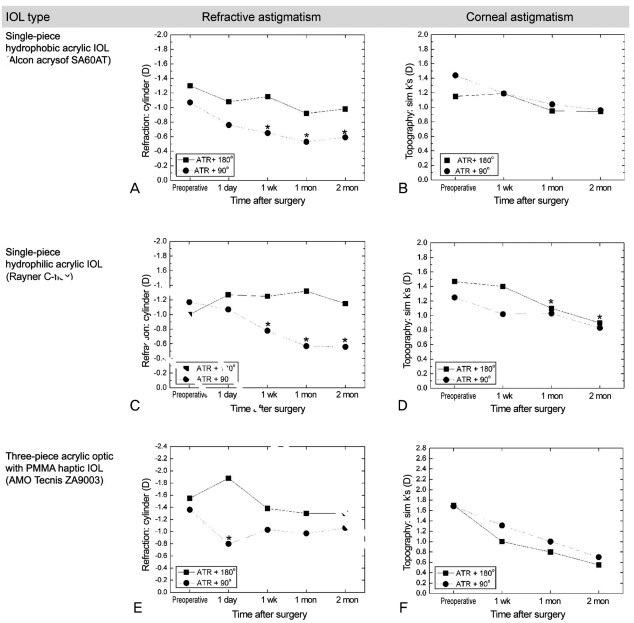
The changes in mean astigmatism by intraocular lens (IOL) axis in against-the-rule (ATR) astigmatic patients. Single-piece hydrophobic acrylic IOL group by refraction (A) and topography (B). Single-piece hydrophilic acrylic IOL group by refraction (C) and topography (D). Three-piece acrylic optic with polymethylmethacrylate (PMMA) haptic group by refraction (E) and topography (F) (*p < 0.05 indicates p-value compared with the preoperative values).
D = diopters.
In the second sub-group (a single-piece hydrophilic acrylic IOL), there was a significant difference in refractive astigmatism 1 week, 1 month, and 2 months post-operatively. Specifically, the refractive astigmatisms were 1.25 ± 0.41 D, 1.32 ± 0.51 D, and 1.15 ± 0.40 D in insertion of the IOL at the 180° haptic axis and 0.78 ± 0.74 D, 0.57 ± 0.50 D, and 0.56 ± 0.50 D in insertion of the IOL at the 90° haptic axis, respectively (p = 0.017, 0.002, and 0.008, respectively). Also, there was no difference in corneal astigmatism between IOL insertion at the 180° or 90° haptic axis. Compared with pre-operative astigmatism, there were no differences in corneal astigmatism (Fig. 3D), but refractive astigmatism differed 1 week, 1 month, and 2 months post-operatively (Fig. 3C, indicated as * in the figure).
In the last sub-group (three-piece hydrophobic acrylic optic and a PMMA haptic IOL), there was a significant difference in refractive astigmatism 1 day post-operatively between insertion of the IOL at the 180° and 90° haptic axis. There was also a significant decrease compared to the pre-operative astigmatism 1 day post-operatively in the group with the IOL inserted at the 90° haptic axis (Fig. 3E, indicated as * in the figure). There were no corneal astigmatism differences throughout the post-operative period between the two IOL axes or compared to the pre-operative astigmatism (Fig. 3F).
Discussion
The aim of modern cataract surgery is to achieve optimum uncorrected visual acuity (UCVA). A significant astigmatism, whether preoperative or induced by the surgical procedure, can limit postoperative UCVA. Most methods to reduce pre-existing astigmatism have focused on correcting corneal astigmatism, which accounts for most of the total astigmatism. Most surgeons still prefer superior or temporal approaches, while a few adopt an "on-axis" approach, placing the incision on the steeper corneal meridian when the preoperative corneal astigmatism is significant.
Recently developed toric IOLs can correct astigmatism up to 3.0 D. Choosing the amount of astigmatism to be corrected by a toric IOL is based on the corneal astigmatism, which is measured by keratometry or topography. Even after implantation of a toric IOL, however, ocular residual astigmatism remains. This component of astigmatism is not considered when the toric IOL is chosen.
Dunne et al. [9] reported that ocular residual astigmatism is approximately 0.5 D with 66% to 83% exhibiting ATR astigmatism. This ocular residual astigmatism does not resolve, even after cataract surgery [19,20]. Bae et al. [21]. reported that the pseudophakic eye has 0.47 D of ATR ocular residual astigmatism. This ocular residual astigmatism in the pseudophakic eye is assumed to be due to the left posterior capsule or the IOL.
Temporal clear corneal incisions cause a reduction of astigmatism on average by flattening the horizontal corneal axis and are beneficial because ATR astigmatism is more common in the cataract age group [2]. However, temporal incisions often induce an increase in astigmatism in WTR astigmatic patients. As a result, we determined a way to minimize SIA when performing cataract surgery in WTR patients through a temporal incision. This led us to determine the effect of IOL haptic positioning on astigmatism. In a previous report, we described the method of suturing the temporal incision and insertion of the IOL at the 180° haptic axis in WTR astigmatic patients, and showed that it reduced pre-existing astigmatism and SIA during clear corneal temporal incision cataract surgery [22]. The report also showed that greater astigmatic reduction was achieved in the group with insertion of the IOL at the 180° haptic axis than the group with insertion of the IOL at the 90° haptic axis. This phenomenon of astigmatism reduction according to the haptic position of the IOL has been further studied. In terms of ocular residual astigmatism, refractive changes induced by IOL tilt and longitudinal displacement are well known; this being the case, we wondered whether IOL positioning in the capsule bag could have an affect on postoperative astigmatism in cataract surgery. Therefore, astigmatic outcomes by haptic axis (vertical vs. horizontal) of the IOL in WTR and ATR astigmatism patients during cataract surgery were evaluated.
In WTR patients, insertion of the IOL at the 90° haptic axis (group 2) resulted in a 0.35 ± 0.22 D increase of corneal astigmatism due to the effect of temporal corneal incision. The insertion of the IOL at the 180° haptic axis (group 1) resulted in a 0.22 ± 0.53 D decrease with decreased refractive astigmatism. This affect may have been due to the IOL insertion axis (Fig. 2A and 2B). In ATR patients, insertion of the IOL at the 180° haptic axis (group 3) decreased corneal astigmatism by 0.23 ± 0.93 D due to the effect of temporal corneal incision. Insertion of the IOL at the 90° haptic axis (group 4) exhibited a 0.40 ± 0.68 D decrease with decreased refractive astigmatism (Fig. 2C and 2D).
This phenomenon may be explained by IOL angulation that may develop according to the long axis of the IOL when the IOL is inserted into the capsular bag. The total IOL length is longer than the capsular bag diameter, which is approximately 10.38 ± 0.35 mm [23,24]. This IOL angulation may stretch the posterior capsule and stiffen it at the inserted axis. This induces astigmatism perpendicular to the inserted axis. When the inserted IOL axis is parallel to the preoperative astigmatism axis, the induced astigmatism may counterbalance the total astigmatism, which in turn reduces the total astigmatism [22]. This result was consistent with the polar value analysis (Table 1). In group 1, SIA decreased by 0.30 ± 0.17 D, and this was statistically significant when comparing the pre-operative and post-operative AKP values. In group 4, SIA decreased by 0.36 ± 0.12 D, and this was also statistically significant.
Analyzing the data in ATR patients based on the different types of IOLs yielded similar results. All 3 types of IOLs had total overall lengths larger than the capsular bag diameter. In the single-piece hydrophobic acrylic IOL and single-piece hydrophilic acrylic IOL group, the axis of the inserted IOL at 180° or 90° showed no differences in corneal astigmatism. However, refractive astigmatism showed a significant difference from the haptic axis 1 week, 1 month, and 2 months post-operatively (Fig. 3A-3D). In the three-piece PMMA haptic IOL group, refractive astigmatism only showed a significant difference 1 week post-operatively (Fig. 3E and 3F). This observation might be attributed to the material and the properties of the IOL with the acrylic IOL being softer and more flexible than the three-piece PMMA haptic IOL. When inserted into the capsule bag, the acrylic IOL will angulate more easily and induce greater stretching of the posterior capsule. This will create more astigmatism and in turn, result in a greater significant difference of refractive astigmatism by the haptic axis at 180° or 90°.
The clinical data from our study may be not sufficient to conclude that the axis change of inserted IOLs can reduce refractive astigmatism. However, it was determined that there is a tendency and possibility that IOL positioning in the capsule bag could affect postoperative astigmatism in cataract surgery. As a result, we could postulate that changing the axis of inserted IOLs during cataract surgery may have a characteristic effect on astigmatism. Insertion of the IOL at the 180° haptic axis in WTR patients could reduce pre-existing WTR astigmatism, and insertion of the IOL at the 90° haptic axis in ATR patients could reduce ATR astigmatism. This method reduces astigmatism approximately 0.3 D to 0.4 D with a simple procedure that can be performed during cataract surgery. The effect was greater with a one-piece acrylic IOL than a three-piece PMMA haptic IOL. Ocular residual astigmatism after cataract surgery may be important when inserting toric, aspheric, and multifocal IOLs. Thus, the possibility that axis change of inserted IOLs may affect ocular residual astigmatism should be considered when performing cataract surgery.
Notes
This paper was presented as a journal at the ASCRS Symposium in Chicago, IL, USA in April 2008.
No potential conflict of interest relevant to this article was reported.