 |
 |
| Korean J Ophthalmol > Volume 24(6); 2010 > Article |
Abstract
Purpose
To evaluate the clinical features, associated factors, and treatment outcomes of scleritis in the Korean population.
Methods
Medical records were retrospectively reviewed for 94 eyes of 76 patients with scleritis. Clinical features of scleritis, including systemic disease, presence of microorganisms, serologic markers, history of previous ocular surgery, and use of immunosuppressants were investigated and compared amongst the subtypes of scleritis. Treatment outcomes were evaluated using best corrected visual acuity (BCVA) and time to scleritis remission.
Results
Nodular scleritis was the most common form observed, followed by necrotizing scleritis with inflammation, diffuse scleritis, and necrotizing scleritis without inflammation, respectively. A total of 16 of 76 patients (21.1%) had connective tissue diseases. Eleven cases (14.5%) had infectious scleritis, of which bacteria (54.5%) and fungi (45.5%) were the causative microorganisms. Thirty-three patients (43.4%) had previous ocular surgery, mostly pterygium excision. Notably, a history of pterygium excision was significantly associated with development of necrotizing and infectious scleritis (odds ratio [OR], 399 and 10.1; p < 0.001 and 0.002, respectively). In addition, patients with necrotizing scleritis were more likely to have infectious scleritis (OR, 11.7; p = 0.001). BCVA after treatment and time to remission also showed significant differences among the different scleritis subtypes. Systemic immunosuppression was required in addition to steroids for treating diffuse and necrotizing scleritis.
Conclusions
Careful taking of patient history including previous pterygium excision should be performed, especially in patients with necrotizing and infectious scleritis. In addition, evaluation of microbiological infection can be crucial for patients with necrotizing scleritis and history of pterygium excision.
Scleritis is a severe ocular inflammatory disorder that is commonly associated with systemic collagen vascular disease. Previous reports have demonstrated that approximately 50% of patients with scleritis have systemic connective tissue diseases [1-6]. However, scleritis can also be caused by ocular surgery, such as pterygium, cataract extraction, or strabismus surgeries. Although scleritis is a serious postsurgical complication [7-9], clinical characteristics of surgically-induced scleritis or the type of surgery associated have not been extensively investigated.
Infectious and necrotizing scleritis are two subtypes of scleritis with particularly poor prognoses; there are several cases reporting the requirement for either evisceration or enucleation in order to control the infection and inflammation in infectious and necrotizing scleritis [10]. Systemic immunosuppression in necrotizing scleritis and early antibiotic treatment in infectious scleritis are essential for preserving vision and preventing the spreading of infection into adjacent ocular structures [11]. However, predisposing factors for early diagnosis of these two forms of scleritis have not been thoroughly studied. Here, we investigated the clinical characteristics and predisposing factors of scleritis with respect to treatment course and outcome in the Korean population.
Medical records of patients who were diagnosed with scleritis at Seoul National University Hospital between January 2005 and December 2008 were reviewed. The diagnosis of scleritis was made based on the presence of inflammation of scleral and episcleral vessels that did not blanch with topical phenylephrine, the presence of scleral thinning, edema, or nodules. Patients who did not have a follow-up visit were excluded from this study. Scleritis was classified into the following subtypes; diffuse, nodular, and necrotizing scleritis with or without inflammation. Diffuse scleritis was characterized by the presence of scleral edema and redness with a tortuous vascular network. Nodular scleritis presented as a nodule that was firm and tender to palpation. Necrotizing scleritis with inflammation was defined as having an avascular area of sclera with adjacent scleral edema and congestion while necrotizing scleritis without inflammation was classified for cases presenting with a thin and avascular area of sclera with no active neighboring inflammation. Infectious scleritis was diagnosed following isolation of the causative microorganism from the lesion according to a microbiological workup. For first-line treatment for non-infectious nodular or diffuse scleritis without any underlying systemic disease, topical and oral corticosteroids or systemic non-steroidal anti-inflammatory drugs were used. In severe cases refractory to oral corticosteroid therapy, systemic immunosuppressive agents such as azathioprine, cyclosporine, and mycophenolate mofetil were used. For treatment of infectious scleritis, topical and oral antibiotics or anti-fungal agents were used after a causative microorganism was isolated by culture from scleral scrapings in the base of active lesion [9,10].
In total, 94 eyes of 76 patients met the inclusion criteria. The study protocol was approved by the Institutional Review Board, and informed consent was obtained from patients. Patient medical records were retrospectively reviewed with respect to clinical manifestations, subtypes of scleritis, presence of systemic or ocular co-morbidities, and a history of previous ocular surgery. In addition, serologic markers and presence of causative microorganisms isolated from culture from cases of infectious scleritis were investigated. The markers included veneral disease research laboratory, fluorescent anti-nuclear antibody (FANA), anti-neutrophil cytoplasmic antibody (ANCA), rheumatoid factor, human leukocyte antigen-B27, anti-streptolysin O, and ribonucleoprotein antibody. To estimate the significance of the risk factors for the development of infectious and necrotizing scleritis, an odds ratio was calculated for each risk factor.
To compare visual outcomes, best corrected visual acuities (BCVA) were evaluated based on the logarithmic value of the minimal angle of resolution scale at the time of diagnosis and after clinical remission. Additionally, the time elapsed from diagnosis to remission of scleritis was analyzed. Clinical remission was defined as when 1) the patient complained of no symptoms and when 2) any sign of scleral inflammation disappeared on slit-lamp examination. Both BCVA and time to remission were compared between seropositive and seronegative patients as well as infectious and noninfectious using Student's t-test, one-way analysis of variance (ANOVA), and survival analysis.
In order to determine which factors required the use of additional systemic immunosuppression for treating scleritis, patients were divided into two groups consisting of those who were treated with oral corticosteroids only and those who needed additional systemic immunosuppressants for resolution of the disease. The subtypes of scleritis, seropositivity, and systemic comorbidities were then compared between the two groups using Fisher's exact test, likelihood ratio test for categorical data, and Student's t-test.
All statistical analyses were performed using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). A p-value < 0.05 was considered statistically significant.
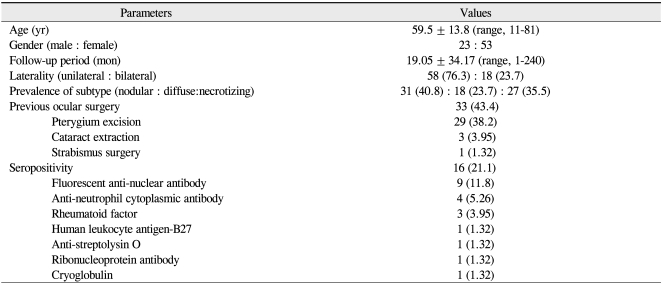
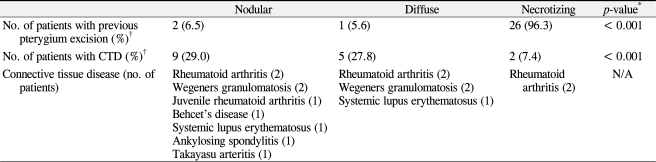
Demographic data are summarized in Table 1. Scleritis was more than twice as times prevalent in females than males (male:female = 23:53), and 76.3% of the cases were unilateral. The most common subtype of scleritis was nodular scleritis (40.8%), followed by necrotizing scleritis with inflammation (27.6%), diffuse scleritis (23.7%), and necrotizing scleritis without inflammation (7.89%). Associated clinical factors are shown in Table 1. Sixteen patients (21.1%) were seropositive, most commonly FANA-positive (n = 9). Notably, 33 out of 76 patients (43.4%) had previously ocular surgeries, of which 29 cases were pterygium excision. Of note was a high association between a history of pterygium surgery and the development of necrotizing or infectious scleritis. Specifically, most of the patients with necrotizing scleritis (96.3%) had a previous history of pterygium surgery; however, only 3 of 49 patients (6.1%) with non-necrotizing scleritis had a prior pterygium excision. Additionally, 89.3% of scleritis cases with a history of pterygium excision were necrotizing scleritis. The odds ratio for a history of pterygium excision was 399 in patients with necrotizing scleritis compared to those with non-necrotizing scleritis (95% confidence interval [CI], 39.4-4,030; p < 0.001). In addition, 9 out of 11 patients (81.8%) with infectious scleritis had a history of prior pterygium surgery. The odds ratio for a history of pterygium excision was 10.1 in infectious scleritis compared to noninfectious scleritis (95% CI, 2.00-51.2; p = 0.002).
Moreover, a significant association between necrotizing and infectious scleritis was observed. Specifically, of the 11 patients with infectious scleritis, 9 (88.8%) were diagnosed with necrotizing scleritis, which resulted in an odds ratio for infectious scleritis of 11.8 for patients with necrotizing compared to non-necrotizing scleritis (95% CI, 2.31-59.7; p = 0.001). Thus, necrotizing scleritis was significantly associated with infectious scleritis and a history of pterygium surgery.
Sixteen patients (21.1%) had connective tissue diseases. The percentage of associated connective tissue diseases was different among the subtypes of scleritis (Table 2). In addition, diffuse and nodular scleritis was associated with comorbidity of connective tissue disease, although statistical significance was borderline (p = 0.07). Rheumatoid arthritis was the most common connective tissue disease associated with scleritis.
Eleven eyes (14.5%) were diagnosed as having infectious scleritis. Bacterial infection comprised 54.5% (n = 6) of these cases, followed by fungi (n = 5, 45.5%) and viruses (n = 1, 9.09%). Pseudomonas was the most common pathogen (3 cases), followed by Fusarium and Stenotrophophilia (2 cases). Haemophilus, Acromobacter, Aspergillus, Beauveria, Candida, and herpes zoster were also isolated. There were two cases of coinfections; one was coinfection of Pseudomonas and Candida, and the other was coinfection of Stenotrophophilia and Acromobacter. Two eyes with fungal scleritis were eviscerated because of uncontrolled infection that eventually progressed into endophthalmitis.
To evaluate visual outcome according to subtypes of scleritis, BCVA was investigated at the time of diagnosis and after remission (Fig. 1). BCVA at the time of diagnosis was worse in patients with infectious and necrotizing scleritis than patients with nodular and diffuse scleritis. Patients with infectious scleritis had the worst BCVA after remission (1.19 ┬▒ 1.11), followed by non-infectious necrotizing (0.538 ┬▒ 0.457), nodular (0.367 ┬▒ 0.637), and diffuse scleritis (0.282 ┬▒ 0.506). These differences in visual acuities among subtypes of scleritis were statistically significant (p = 0.013 at the time of diagnosis and 0.008 after remission; one-way ANOVA).
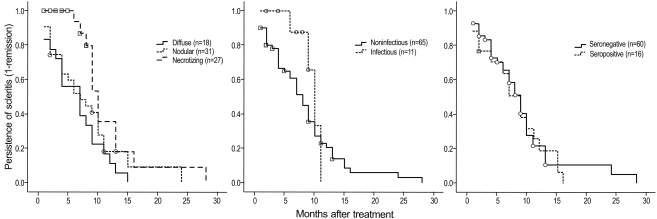
The median time to resolution was significantly longer in necrotizing scleritis (10 months, p = 0.028; log rank test), compared to diffuse (7 months) and nodular subtypes (7 months) (Fig. 2). The median time to resolution of infectious scleritis was longer than non-infectious type (10 months vs. 8 months); however, this difference was not statistically significant (p = 0.166). There was no difference in the median time to resolution between seropositive and seronegative scleritis (p = 0.732).
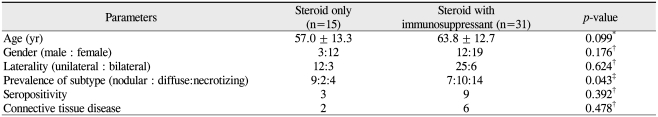
In order to determine the factors associated with the use of additional systemic immunosuppression, we analyzed the clinical characteristics between the patients who were treated with conventional oral corticosteroids and the patients who needed additional systemic immunosuppressants because of inflammation refractory to systemic steroids (Table 3). We found that patients with diffuse and necrotizing scleritis were more likely to require systemic immunosuppression (p = 0.043, likelihood ratio test).
A noticeable finding of this study was that patients with necrotizing scleritis were more likely to have had previous pterygium excision and infectious scleritis. This finding is consistent with the report by O'Donoghue [8], who demonstrated a high association between necrotizing and surgically-induced scleritis. The association between a history of pterygium excision and two destructive subtypes of scleritis (necrotizing and infectious) might suggest a role for pterygium excision as either a causative or inducing factor of the two types of scleritis. This hypothesis can be explained by the previously suggested proposal that surgical techniques or intra- or postoperative medications for pterygium excision might predispose inflammation and necrosis of the sclera and make ocular tissue vulnerable to infection [12]. In addition, it is possible that the use of mitomycin C during pterygium surgery may induce necrotizing and infectious scleritis.
For successful treatment of scleritis patients, early discrimination between noninfectious autoimmune scleritis and infectious scleritis is important since the treatment regimens for infectious scleritis, antibiotics, is contrary to the treatment for non-infectious necrotizing scleritis, which consists of systemic immunosuppressants. However, the differential diagnosis between necrotizing scleritis with and without infection is often difficult. Indeed, two patients in our study whose eyes were eviscerated because of uncontrolled fungal scleritis were initially misdiagnosed as having an immune-mediated necrotizing scleritis and were initially treated with topical and systemic immunosuppressants. Given that pterygium excision was a significant factor associated with both necrotizing and infectious scleritis, careful examination for signs of infection and microbiological work-up should be performed, especially in patients who present with scleral necrosis.
Another remarkable finding of this study was that fungal infection comprised of almost half cases of infectious scleritis in our study. Indeed, the proportion of fungal scleritis in the present study was substantially higher than previously reported [9,10,13]. This may be because of a geographic difference of an infectious pathogen in scleritis as reported in previous studies [9,10,13]. The prognosis of fungal scleritis in this study was poor, as previously reported [14-18].
As shown in the present study, visual outcome depended on the subtypes of scleritis. Nodular or diffuse scleritis showed better visual acuity both at the time of diagnosis and after remission. Necrotizing scleritis, especially when associated with infectious scleritis, demonstrated poor visual acuity both at the time of diagnosis and after remission. In addition, patients with necrotizing scleritis had a prolonged duration of disease. Furthermore, patients with non-infectious necrotizing scleritis were more likely to require systemic immunosuppressants in addition to conventional oral steroids for treatment, compared to those with nonnecrotizing scleritis. An association between the use of additional immunosuppressants and other clinical factors such as ANCA, which was reported in previous studies [19], was not found in this study.
In conclusion, the results of this study suggest that a history of pterygium excision in scleritis is a significant factor associated with poor prognosis due to the strong association with necrotizing and infectious scleritis. Hence, when patients with a history of pterygium surgery present with necrotizing scleritis, immunosuppressants should be cautiously started after excluding the possibility of an infection by a thorough microbiological work-up.
REFERENCES
1. Akpek EK, Thorne JE, Qazi FA, et al. Evaluation of patients with scleritis for systemic disease. Ophthalmology 2004;111:501-506.


2. Sainz de la Maza M, Jabbur NS, Foster CS. Severity of scleritis and episcleritis. Ophthalmology 1994;101:389-396.


5. Sainz de la Maza M, Foster CS, Jabbur NS. Scleritis associated with rheumatoid arthritis and with other systemic immune-mediated diseases. Ophthalmology 1994;101:1281-1286.


6. Erkanli L, Akova YA, Guney-Tefekli E, Tugal-Tutkun I. Clinical features, prognosis, and treatment results of patients with scleritis from 2 tertiary eye care centers in Turkey. Cornea 2010;29:26-33.


7. Lee JE, Oum BS, Choi HY, Lee JS. Methicillin-resistant Staphylococcus aureus sclerokeratitis after pterygium excision. Cornea 2007;26:744-746.


8. O'Donoghue E, Lightman S, Tuft S, Watson P. Surgically induced necrotising sclerokeratitis (SINS): precipitating factors and response to treatment. Br J Ophthalmol 1992;76:17-21.



9. Su CY, Tsai JJ, Chang YC, Lin CP. Immunologic and clinical manifestations of infectious scleritis after pterygium excision. Cornea 2006;25:663-666.


10. Huang FC, Huang SP, Tseng SH. Management of infectious scleritis after pterygium excision. Cornea 2000;19:34-39.


11. Jabs DA, Mudun A, Dunn JP, Marsh MJ. Episcleritis and scleritis: clinical features and treatment results. Am J Ophthalmol 2000;130:469-476.


12. Singh RP, McCluskey P. Scedosporium prolificans sclerokeratitis 10 years after pterygium excision with adjunctive mitomycin C. Clin Experiment Ophthalmol 2005;33:433-434.


13. Jain V, Garg P, Sharma S. Microbial scleritis-experience from a developing country. Eye (Lond) 2009;23:255-261.


14. Hsiao CH, Chen JJ, Huang SC, et al. Intrascleral dissemination of infectious scleritis following pterygium excision. Br J Ophthalmol 1998;82:29-34.



15. Lincoff HA, McLean JM, Nano H. Scleral abscess. I. A complication of retinal detachment. Buckling procedures. Arch Ophthalmol 1965;74:641-648.


16. Margo CE, Polack FM, Hood CI. Aspergillus panophthalmitis complicating treatment of pterygium. Cornea 1988;7:285-289.


17. Milauskas AT, Duke JR. Mycotic scleral abscess. Report of a case following a scleral buckling operation for retinal detachment. Am J Ophthalmol 1967;63:951-954.


Fig.┬Ā1
Visual acuities before and after treatment. Patients with infectious necrotizing scleritis had the poorest best corrected visual acuities (BCVA) before and after treatment. The data are presented as the mean┬▒standard deviation. logMAR = logarithmic value of the minimal angle of resolution.
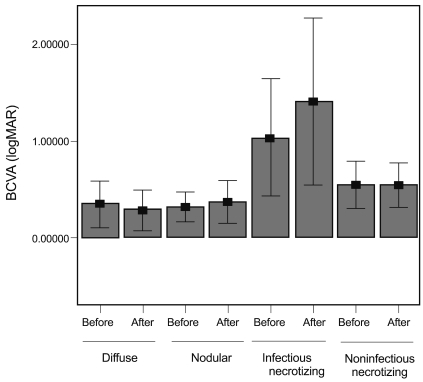
Fig.┬Ā2
Survival curves of remission. A significant difference in remission was observed (p=0.028) among the different scleritis subtypes. No significant difference was seen between infectious versus noninfectious, and seropositive versus seronegative cases. The y-axis indicates the proportion of cases with persistent scleritis as calculated by the following equation: (1 - the proportion of remission).
Table┬Ā1
Demographics, clinical features, previous ocular surgery, and seropositive cases of scleritis



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




