Eye-Preserving Therapy in Retinoblastoma: Prolonged Primary Chemotherapy Alone or Combined with Local Therapy
Article information
Abstract
Purpose
To evaluate the efficacy of primary chemotherapy combined with local therapy in the treatment of retinoblastomas not treatable with a single therapeutic method.
Methods
We performed a retrospective chart review of 227 patients diagnosed with retinoblastoma. Sixty-five eyes in 52 patients had tumors not treatable with a single therapeutic method and received primary chemotherapy combined with local therapy as needed.
Results
Tumor control and eye salvage was achieved in 34 of the 65 eyes; the probability of ocular survival was 46.56% using the Kaplan-Meier method. Forty-three of the 65 eyes were group D or E tumors, in which tumor control and eye salvage was achieved in 16 eyes. Twenty eyes were treated with chemotherapy only, while 28 eyes received one additional modality of local therapy, and 17 eyes received two modalities of local therapy. Of the eyes treated with chemotherapy only, tumor control was achieved in 5 eyes.
Conclusions
Primary chemotherapy combined with local therapy can be effective and safe in the treatment of retinoblastomas otherwise untreatable with other therapeutic methods, such as group D and E retinoblastomas. More vigorous treatment with more local therapeutic methods combined may yield even better results.
Retinoblastoma is a childhood tumor that has a fairly good prognosis. Being the most common intraocular malignant tumor in childhood, the overall survival rate approaches 95%, with enucleation and external beam radiation therapy (EBRT) being the most effective methods for eradicating the tumor [1]. Therefore, patients and doctors have recently begun considering salvage of the eye and vision in addition to just the survival of the patient.
Previously, the standard method of treatment for unilateral retinoblastoma was enucleation. For bilateral retinoblastomas, enucleation of the eye with the more advanced tumor and EBRT of the other eye was the standard therapy [2]. Enucleation has the disadvantages of total loss of both the eye and vision; EBRT is also not without problems given the occurrence of secondary malignancies [3] and disfigurement of the treated orbit [3-5]. As salvage of the eye and vision has become an increasingly important issue in addition to patient survival, there has been an increasing interest in eye-preserving therapeutic methods such as primary chemotherapy, laser photocoagulation, cryotherapy, thermotherapy, and brachytherapy. These methods have previously yielded poor outcomes when used as a sole therapy. Local therapeutic methods have not been able to control the tumor completely and their usage is limited to small tumors in certain locations. Chemotherapy used alone as a primary method of treatment has also revealed disappointing results in the past. In our previous study with prolonged primary chemotherapy for at least 13 cycles, tumors regressed in size initially but then remained stable [6]. This made it difficult to obtain total regression of large tumors. Prolonged primary chemotherapy could be considered as an alternative treatment in tumors classified as Reese-Ellsworth classification (R-E classification) III [7] or less, but should not be considered as a useful treatment in high-stage retinoblastoma. Recently, chemotherapy has been noted to be useful as an adjuvant method to reduce tumors too large to treat with local therapeutic methods to a more manageable size [2,8-15]. Although this has increased the range of tumors that can be managed with local therapeutic methods, more advanced tumors still have no alternative definitive treatment other than enucleation or EBRT. The treatment of such tumors remains challenging and many combinations of various methods are being investigated.
In this study, we combined local therapeutic methods such as thermotherapy, cryotherapy, and laser photocoagulation with our previously studied prolonged primary chemotherapy regimen for the treatment of retinoblastomas that were not treatable with a single therapeutic method.
Materials and Methods
The medical records of 227 patients diagnosed with retinoblastoma at Seoul National University Children's Hospital between March 1997 and September 2007 were retrospectively studied. The diagnosis of retinoblastoma was based on indirect ophthalmoscopy, computed tomography, and ultrasonography findings. The stage was assessed with R-E classification and the International Classification of Retinoblastoma (ICR) [16,17]. Upon diagnosis of retinoblastoma, a brain magnetic resonance imaging and bone scan were performed to evaluate systemic metastasis.
Of the patients diagnosed with retinoblastoma, patients who had been treated with primary chemotherapy with or without the combination of various local therapeutic methods were selected and included in this study. Patients whose tumors were small and able to be treated with local therapeutic methods alone were not included. Patients with documented evidence of systemic metastasis or with evidence of extraocular extension at diagnosis were also excluded. Patients who had chosen to undergo primary enucleation after thorough discussion of the available treatment options and their prognoses were also not included in this study.
Chemotherapy
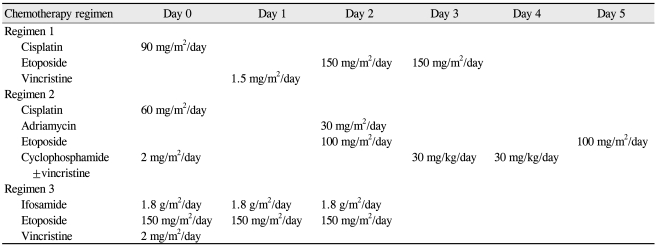
The chemotherapeutic regimen consisted of a 13-cycle combination of cisplatin (carboplatin in some patients), etoposide, and vincristine, which is used in the treatment of central nervous system tumors [8,18-20]. An ophthalmoscopic examination was done either on an outpatient basis or under general anesthesia at the beginning of each cycle according to the patient's condition. Additionally, every patient was evaluated by a pediatric oncologist regularly for possible adverse effects. The chemotherapy regimen was changed to regimen 2 if the tumor showed a minimal response to the basic regimen or if adverse effects, such as nephrotoxicity, were detected. The chemotherapy regimen was changed to regimen 3 if the treatment response continued to be minimal and if growth of the tumor or new lesions were detected despite treatment either with the basic regimen or regimen 2. The regimen was also modified according to the patients' general condition and if any adverse effect of a specific chemotherapeutic agent was suspected. Chemotherapy regimens and dosages used in this study are noted in Table 1.
Local therapy
Local therapy such as thermotherapy, cryotherapy, and laser photocoagulation was added depending on the status of each tumor after each cycle of chemotherapy. Selection of the local treatment method was based on tumor size and location [21]. Thermotherapy was done in patients with tumors with a base less than 3 mm and a thickness less than 3 mm without vitreous seeds located posterior to the equator. Laser photocoagulation was done in similar cases, but also in slightly larger tumors by surrounding the tumor and closing off feeding vessels. Cryotherapy was done in lesions similar in size but located anterior to the equator. Brachytherapy was not available and was not used in this study.
The patients were grouped according to tumor classification (R-E classification, ICR), therapeutic methods used, and the status of the contralateral eye. Treatment outcome was assessed as whether or not enucleation was required. Enucleation was performed in eyes with tumors that showed persistent viable appearance with no signs of regression or in eyes with newly appearing lesions after two or more cycles of treatment. Enucleation was also performed in eyes with media opacities rendering their tumors impossible to follow. The patients in which tumors had totally regressed and therefore avoided enucleation were considered to have had a favorable outcome. EBRT was not performed as a salvage treatment due to the superior efficacy of enucleation in tumor control and the possible occurrence of secondary malignancy and disfigurement of the orbit.
Informed consent was obtained from every patient at the time of diagnosis and initiation of treatment and also before the beginning of each cycle of chemotherapy.
Results
Of the 227 patients diagnosed with retinoblastoma in our institution, a total of 52 patients, 65 eyes were included in this study. The demographics of the included patients are summarized in Table 2. The mean age at diagnosis of retinoblastoma was 13.9 months. All patients presented with leukocoria or strabismus. The mean period of follow-up after diagnosis was 53.33 months. The mean number of chemotherapy cycles completed was 13.37 cycles per patient.
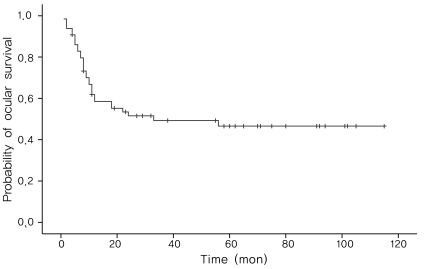
Overall, 34 eyes achieved total regression of the tumor and were able to avoid enucleation. The probability of ocular survival was 46.56±6.70% using the Kaplan-Meier method (Fig. 1). Treatment outcomes of patients according to the R-E classification and ICR are shown in Table 3.

Treatment outcome of patients according to the Reese-Ellsworth classification (R-E classification) and International Classification of Retinoblastoma (ICR)
A relatively similar number of patients were in each group of R-E classification, but the majority of the eyes included in this study were in group D according to the ICR. The majority of eyes with tumors of group C or lower were excluded from the study because they were eligible for local therapy alone. The eyes with group A and B tumors that were included in this study had undergone primary chemotherapy because of a group D tumor in the contralateral eye.
There was a marked difference in the treatment outcome among patients classified as R-E group I to II and R-E group III to V. In patients with R-E classification I and II tumors, complete remission of the tumors was observed in 100% and 86.7% of patients, respectively. In contrast, only 33.3%, 40%, and 20% of the R-E classification III, IV, and V group patients avoided enucleation, respectively. In ICR group D tumor eyes, 35.7% achieved successful regression of the tumor.
The local modalities of treatment used in this study included cryotherapy, thermotherapy, and laser photocoagulation. Twenty eyes in 18 patients received chemotherapy only, while 28 eyes in 21 patients were treated with chemotherapy and one modality of local therapy. Seventeen eyes in 13 patients were treated with chemotherapy and two modalities of local therapy. No patients were treated with chemotherapy and all three modalities of local therapy. Treatment outcomes and the number of local modalities used is shown in Table 4. Five of the 20 eyes treated with chemotherapy only achieved tumor control.
The treatment outcome of patients with unilateral and bilateral involvement is shown in Table 5. Twenty-three patients had unilateral involvement. Sixteen patients had bilateral involvement with extensive involvement in one eye requiring primary enucleation. Thirteen other patients had bilateral involvement but did not have to undergo primary enucleation. Three out of 23 eyes with unilateral involvement were saved while 13 out of the 16 eyes of patients with bilateral involvement who had undergone primary enucleation of one eye were saved. Eighteen of the 26 eyes in patients with bilateral involvement and no enucleation were saved. Tumors in the eyes with bilateral involvement and enucleation of the more severe eye had a significantly lower R-E classification (p=0.001).
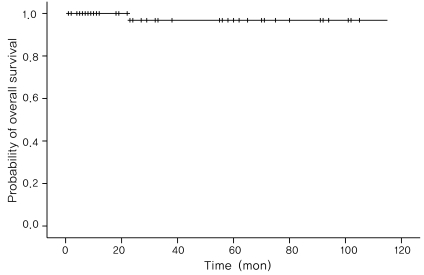
Complications of the therapy included mild and manageable complications such as myelosupression, neutropenic fever, alopecia, mild nephrotoxicity, and ileus. Only one case was fatal, which was due to Fanconi syndrome. The probability of overall survival was 96.77±3.17% using the Kaplan-Meier method (Fig. 2).
There was one case of hyphema and vitreous hemorrhage and one case of cataract, which was thought to be due to local therapy, but the direct relationship remains uncertain. In both cases enucleation was performed due to the limited ability for assessment of tumor progression.
Discussion
Primary chemotherapy, when combined with local therapy, showed a probability of ocular survival of 46.56% in retinoblastomas not treatable with local therapy alone. Forty-three cases (66.2%) in this study were group D or E tumors, and 16 of these 43 eyes (37.2%) avoided enucleation.
Eye-preserving treatment varies widely throughout the world. There have been many studies evaluating the results of these various treatment methods. Systemic chemoreduction followed by local therapy has shown various tumor control rates up to 78 to 85% [12,14,15]. However, when comparing the treatment outcomes of these studies, the severity of the tumors in the subjects included in the studies should be taken into consideration. These studies usually have a heterogeneous severity of tumors included, often including a fair proportion of tumors of low severity. Our study included only tumors that were considered otherwise untreatable with conventional treatment methods, which renders direct comparison of these previous survival rates with our results irrational.
Recently, successful tumor control has even been reported with chemotherapy alone in more advanced tumors. Cohen et al. [22] reported results of chemotherapy (with six cycles of vincristine, etoposide, and carboblatin) in group D heritable retinoblastoma. Successful tumor control with chemotherapy was obtained in only 2 eyes (11%), although 9 more eyes (50%) underwent successful salvage treatment with EBRT. Shields et al. [23] reported results of chemoreduction with 6 cycles of vincristine, etoposide, and carboplatin with or without prophylactic low-dose EBRT for group E retinoblastoma, with a globe salvage rate of 20/42 (48%) in the chemoreduction only group and 4/5 (80%) in the chemoreduction and prophylactic low-dose EBRT group. Our results of a globe salvage rate of 37.2% in group D or E tumors without salvage treatment with EBRT is comparable to the results of previous studies. This treatment method can be considered an effective method with minimal complications; it is especially valuable in bilateral cases when preservation of the eye and vision is more critical, or in cases when a patient strongly refuses enucleation.
There was a tendency towards a better outcome in patients who were treated with more local treatment modalities. The salvage rate was 3/23 (13.0%) in unilateral cases, compared to a salvage rate of 18/26 (69.3%) in bilateral cases, indicating that there was a tendency towards enucleation in unilateral cases. This may have been due to a predilection to completely eradicate the tumor when even the slightest amount of evidence of progression was present. These findings suggest that with more vigorous treatment of the tumor with all possible additional local therapeutic methods, the overall eyeball salvage rate could be even higher.
There are many shortcomings of this study due to the low prevalence of retinoblastoma. The sample size was small and this study was a nonrandomized, retrospective study. Also, the local treatment modalities were selected according to the individual patient's conditions and were not standardized. The selected secondary chemotherapy regimens were also not standardized. However, many of these shortcomings also apply to other studies regarding the treatment of retinoblastoma.
In conclusion, chemoreduction and local therapy can be considered as a first-line treatment in low grade tumors. In higher grade tumors, although enucleation is still the treatment of choice in the most severe cases with vitreous seeding or massive tumors involving over half the retina, chemotherapy combined with local therapy can also be given as a choice of treatment to parents in intermediate cases, especially in bilateral cases with one eye already requiring enucleation. Further study is necessary to determine the most effective chemotherapy regimen and local therapy protocol. The tendency of a better outcome in patients treated with more modalities of local therapy requires further study.
Notes
No potential conflict of interest relevant to this article was reported.