Clinical Outcomes of Ahmed Glaucoma Valve Implantation Using Tube Ligation and Removable External Stents
Article information
Abstract
Purpose
To investigate the immediate and long-term outcomes of Ahmed glaucoma valve (AGV) implantation with silicone tube ligation and removable external stents.
Methods
This retrospective non-comparative study investigated the outcomes of AGV implantation with silicone tube ligation and removable external stents in 95 eyes (90 patients) with at least 12 months of postoperative follow-up. Qualified success was defined as an intraocular pressure (IOP) of ≤21 mmHg and ≥6 mmHg regardless of anti-glaucoma medication. Those who required additional glaucoma surgery, implant removal or who had phthisis bulbi were considered failures. Hypotony was defined as an IOP of <6 mmHg.
Results
Mean IOP reduced from 37.1±9.7 mmHg preoperatively to 15.2±5.6 mmHg at 12 months postoperatively (p<0.001). Qualified success was achieved in 84.2% at 1 year. Hypotony with an IOP of <6 mmHg was seen in 8.4% and an IOP of <5 mmHg in 3.2% on the first postoperative day. No case of hypotony required surgical intervention. Suprachoroidal hemorrhage did not occur in this study. When stents were removed on the first postoperative day because of an insufficient IOP decrease, the mean IOP decreased significantly from 42.0 mmHg to 14.1 mmHg (p<0.001) after 1 hour. The most common complication was hyphema, which occurred in 17.9%.
Conclusions
Hypotony-related early complications requiring surgical intervention were reduced by ligation and external stents in the tube. In addition, early postoperative high IOPs were managed by removing external stents. The described method can prevent postoperative hypotony after AGV implantation and showed long-term success rates comparable to those reported previously.
Glaucoma drainage devices are generally used to control intraocular pressure (IOP) in refractory glaucoma1 and can lower IOP by allowing aqueous humor to be drained to an extrascleral reservoir through a small-caliber tube. Earlier glaucoma drainage devices have low resistance to aqueous outflow and cause hypotony during the immediate postoperative period until a fibrous capsule develops around the extrascleral plate.2-4
To overcome early postoperative hypotony and its attendant complications, valved implants have been developed, which are designed to restrict excessive aqueous drainage. The Ahmed glaucoma valve (AGV) implant (New World Medical, Inc. Rancho Cucamonga, LA, USA) is a valved implant that was approved by the United States Food and Drug Administration in 1993.5
The AGV implant is designed to open when the IOP is between 8 mmHg and 10 mmHg, and thus maintains an IOP of 8 mmHg or higher.5-7 Moreover, AGV implantation showed a lower incidence of postoperative hypotony than non-valved glaucoma drainage device implantation, but the clinical problem of postoperative hypotony was not resolved by valved glaucoma drainage devices.5,8-12 Previous in vitro tests have shown that valved implants may not completely close after initial perfusion with fluid, and that they may function as flow-restricting devices without demonstrable opening or closing pressures.6,7 Partial ligation of the AGV tube has been attempted to lower early postoperative hypotony and its surgical complications.13,14
Here, we describe the use of a ligated silicone tube with external stents in AGV implant surgery. The tube ligation was considered to restrict flow from the anterior chamber. External stents were added to be easily removed in cases of high IOP during the early postoperative period before the ligated suture was absorbed. In this study we aimed to investigate the immediate and long-term outcomes, in terms of IOP and complications, in AGV implantation using tube ligation and external stents.
Materials and Methods
A retrospective chart review was conducted on consecutive patients who underwent AGV implantation by a single surgeon (K.H.P.) at Seoul National University Hospital from January, 2001 to July, 2004. This study was approved by the hospital Institutional Review Board as a retrospective chart review study which does not require informed consent from the patients. All 95 eyes of 90 patients with a minimum of 12 months of follow-up were included. The following information was gathered from the charts: age, gender, race, eye laterality, glaucoma diagnosis, previous operation history, visual acuity, IOP, number of antiglaucoma medications, and postoperative complications.
After administering retrobulbar and facial nerve blocks, the affected eye was prepared with povidone-iodine and draped. A 6-0 black silk suture was placed through the midstromal cornea at the 12 o'clock position and used for traction. A fornix-based conjunctival flap was then created between two adjacent recti (superotemporal or nasal), the recti were identified, but not isolated. The AGV (model S2) was then irrigated with a balanced salt solution to prime the valve mechanism. A limbus-based scleral flap was created with a rectangle of 4×4 mm. The plate was placed and fixed to the sclera with 8-0 Nylon sutures 8 to 9 mm posterior to the limbus. As an external stent, 3 strands of 8-0 Nylon were put just beside the silicone tube and the ligation of both tube and stent was done with 8-0 Vicryl. The ligature was tightened until the internal lumen of the silicone tube collapsed (Fig. 1). The tube was then positioned over the cornea to measure the required length so as not to cross the pupil margin, and its tip was trimmed so that the bevel on the tip faced the corneal endothelial surface. The anterior chamber was entered through the limbus under the scleral flap with a 23-gauge needle. The AGV tube was then placed into the anterior chamber parallel to the iris plane through the needle track. The scleral flap was then sutured to cover the AGV tube. The conjunctiva and Tenon's capsule were closed with 8-0 Vicryl sutures. No anti-metabolites were used intra- or postoperatively. The external stents consisting of 3 strands of 8-0 Nylon were exposed through the conjunctival wound. The exposed stents could be removed with a forceps during slit lamp examination between 1 day and 1 month after AGV implant surgery. The lengths of the exposed Nylon strands were about 5 mm to prevent corneal irritation.
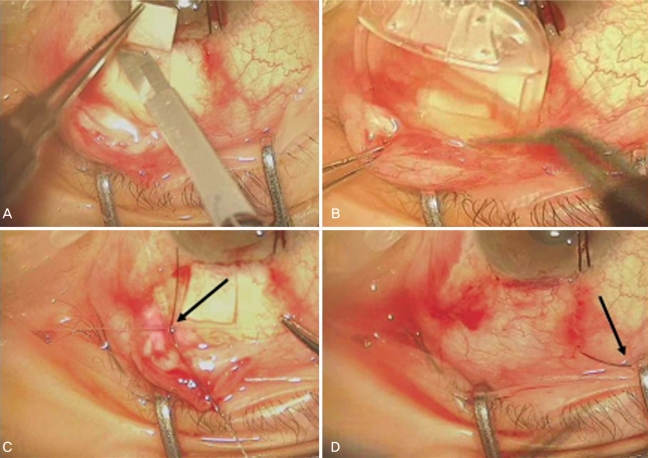
Ahmed glaucoma valve implant surgery with ligation of silicone tube and external stents. (A) A limbus-based scleral flap was created with a Beaver knife. (B) The AGV plate was placed in the sub-Tenon's space in the supero-temporal quadrant. (C) The silicone tube and 3 strands of 8-0 Nylon were ligated with 8-0 Vicryl, and the ligature was tightened until the internal lumen of the tube collapsed. (D) The lengths of the exposed 8-0 Nylon strands were kept to about 5 mm to minimize corneal irritation.
If the IOP did not reduce immediately after surgery, the stents were removed earlier, either on the first postoperative day or at 1 week. Stents were usually removed on the first day when the postoperative IOP was about 25 mmHg or greater. If the postoperative IOP was about 30% less than the preoperative IOP or less than 25 mmHg, stent removal was postponed until 1 week postoperatively.
We assessed surgical outcomes in terms of postoperative IOP and the incidence of complications. Complete success was defined as an IOP of ≤21 mmHg and ≥6 mmHg without additional glaucoma surgery and without anti-glaucoma medication. Qualified success was defined as an IOP of ≤21 mmHg and ≥6 mmHg regardless of the use of anti-glaucoma medication. Failure was defined as an IOP of >21 mmHg on maximally tolerated medication or <6 mmHg. Those who required additional glaucoma surgery, implant removal, or who had phthisis bulbi were also considered failures. Hypotony was defined as an IOP of <6 mmHg. The hypertensive phase (HP) was defined as that period when the IOP rose to >21 mmHg during the first 3 postoperative months following an IOP reduction to ≤21 mmHg.10 Resolution of the HP was evaluated by comparing IOPs and the number of medications needed between 6 to 8 months postoperatively and 1 month following the maximal IOP. Resolution of HP was defined as an IOP of ≤21 mmHg in conjunction with the following: a reduction of IOP by ≥3 mmHg on the same number of medications or less, or a reduction in at least one medication associated with an IOP change of <3 mmHg.10
For comparisons between two normally distributed variables, we used paired t tests, and to compare paired non-normally distributed numeric variables we used the Wilcoxon signed rank test. Additionally, the cumulative probability of success was evaluated by Kaplan-Meier survival analysis during the follow-up period for more than 12 months postoperatively. IOP data are expressed as a mean and standard deviation. Two-tailed p values of <0.05 were considered statistically significant. Statistical analysis was conducted using SPSS for Windows (version 12.0, SPSS Inc., Chicago, IL).
Results
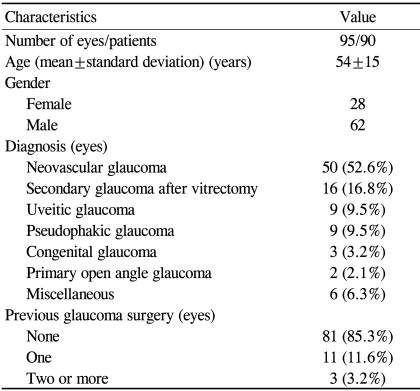
A total of 95 eyes (90 Korean patients) were included in this study. Table 1 summarizes the patient characteristics. The postoperative follow-up period was at least 12 months in each patient (mean follow-up period: 20.3 months). The most common indications for surgery were neovascular glaucoma (NVG) (52.6%) and secondary glaucoma after pars planar vitrectomy (16.8%). 81 eyes (85.3%) did not have any kind of glaucoma surgery before AGV implant surgery. These eyes had refractory glaucoma in which trabeculectomy with adjunctive anti-fibrotic therapy was expected to have a low chance of success in terms of IOP control.15
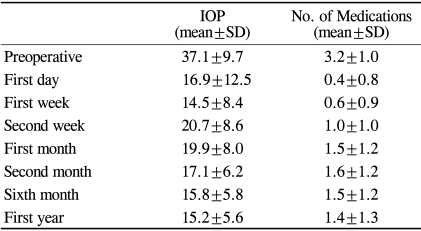
The mean preoperative IOP was 37.1±9.7 mmHg, and the mean postoperative IOP was 16.9±12.5 mmHg on the first postoperative day and 15.2±5.6 mmHg at 12 months (Fig 2, Table 2). The reduction in IOP over the first 12 months of follow-up was statistically significant (p<0.001). T tests for paired data showed a significant IOP reduction from the preoperative baseline at 1 day postoperatively (p<0.001), a significant increase from 1 week to 2 weeks PO (p<0.001), and from 1 week to 1 month postoperatively (p<0.001). A significant decrease also occurred between 1 and 2 months postoperatively (p<0.001).
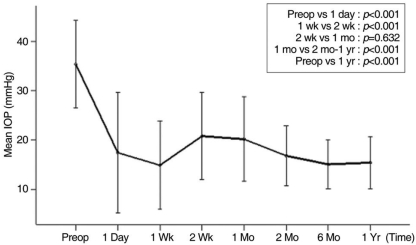
The mean intraocular pressure (mean±standard deviation) after Ahmed glaucoma valve implantation. The hypertensive phase occurred between 2 weeks and 1 month postoperatively. Paired t tests were used to compare the mean intraocular pressures at different time points.
The mean number of anti-glaucoma medications also significantly reduced during the initial postoperative phase and then gradually increased during the first postoperative year (Fig. 3, Table 2). The mean number of anti-glaucoma medications was 3.2 preoperatively, 0.6 at 1 week, 1.0 at 2 weeks, 1.5 at 1 month, and 1.4 at 1 year postoperatively.
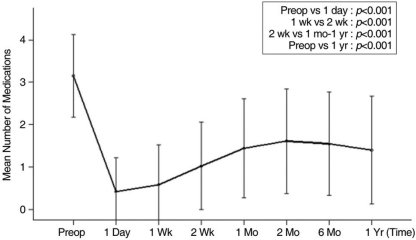
Mean numbers of anti-glaucoma medications (mean±standard deviation) after Ahmed glaucoma valve implantation. Paired t tests were conducted to compare the mean number of medications administered at different time points.
Hypotony with an IOP of <6 mmHg was seen in 8 eyes (8.4%) and an IOP of <5 mmHg was seen in 3 eyes (3.2%) on the first postoperative day before stent removal. The mean IOP of hypotonic eyes with an IOP of <6 mmHg was 4.1 mmHg on the first postoperative day. Hypotony with an IOP of <6 mmHg was seen in 2 eyes (2.1%) at 1 week. All eyes had an IOP of ≥6 mmHg at 2 weeks postoperatively.
An inadequate IOP decrease (IOP>21 mmHg) was detected in 25 eyes (26.3%) on the first postoperative day with a mean IOP in these eyes of 34.9 mmHg. Stent removal was performed in 16 eyes (16.8%) on the first day, in 5 eyes (5.3%) at 1 week, and 4 eyes thereafter. IOP-lowering drugs were added for 9 eyes (9.5%) after stent removal. IOP was controlled with medication only in 2 eyes (2.1%), and 2 eyes (2.1%) showed a normal IOP spontaneously at 1 week postoperatively. IOP on the visit after stent removal was in the normal range, except in one eye. No wound leakage was observed after stent removal.
A record of IOP change before and 1 hour after stent removal was available for 17 eyes. Twelve eyes underwent stent removal on the first postoperative day, with a mean IOP before and 1 hour after stent removal of 42.0±7.9 mmHg and 14.1±7.6 mmHg, respectively, and this IOP reduction after stent removal was statistically significant (Wilcoxon signed rank test, p=0.002). At 1 week, the mean IOP decreased from 34.8±8.9 mmHg to 21.8±12.4 mmHg at 1 hour after stent removal (in 5 eyes), with borderline significance (p=0.068) (Table 3). The three strands of the external stents were removed en bloc in most cases for rapid IOP control. Serial stent removal was done in only two eyes. At first, one strand was removed on postoperative day one, and the IOP was measured 30 minutes after removal. The IOP was reduced from 45 mmHg to 39 mmHg in one eye and from 29 mmHg to 25 mmHg in the other eye. Due to an insufficient IOP decrease, additional stent removal was continued promptly. The IOP finally decreased to 7 mmHg (45→39→7) and 12 mmHg (29→25→12), respectively, after complete stent removal.
The HP was observed in 49 eyes (51.6%) based on the definition given above. In 25 eyes (26.3%), the occurrence of the HP could not be ascertained definitely due to the use of medications, despite an IOP of 21 mmHg or less. The HP appeared after an average time of 2.7±1.5 weeks. The highest IOP in these eyes with a HP ranged from 22 to 48 mmHg, with a mean of 29.8±6.9 mmHg. HP resolution occurred in 38 of the 49 eyes (77.6%), according to the study definition. In most patients that experienced a HP, the IOP was controlled by additional medication. Needling of the bleb was done in 2 eyes, and a second implant was required in three.
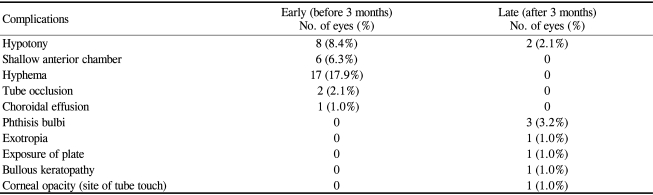
Complications occurred in 33 (34.7%) of 95 eyes after AGV implantation (Table 4). Eight eyes (8.4%) had more than one complication. Complications during the early postoperative period (within 3 months of AGV surgery) were hyphema, postoperative hypotony, a shallow anterior chamber, tube occlusion, and choroidal effusion. Late hypotony, phthisis, exotropia, plate exposure, bullous keratopathy, and corneal opacity were later complications and were encountered after 3 months postoperatively.
The most common complication was hyphema in 17 eyes (17.9%). 88.2% (15 eyes) of these hyphema cases occurred in patients with NVG. Six eyes (6.3%) showed a shallow anterior chamber on the first postoperative day. The mean IOP of these 6 eyes was 9.5 mmHg, and one eye had hypotony. Suprachoroidal hemorrhage did not occur. Choroidal effusion occurred in one eye with congenital glaucoma and an IOP of 6 mmHg, and was treated with oral prednisolone. Tube occlusion was observed in two eyes. These were caused by a blood clot and a vitreous strand in two pseudophakic glaucoma patients. A Nd:YAG laser was used on the tube occluded with the vitreous strand due to a high IOP.
Late hypotony occurred 6 months postoperatively and lasted for more than 3 months in 2 eyes (2.1%). These eyes had shown a normal range of IOPs before the development of late hypotony, without a history of early postoperative hypotony. Phthisis occurred in 3 eyes (3.2%). All 3 of these eyes were diagnosed as NVG preoperatively. At the time of AGV implantation, 1 eye had had a chronic total retinal detachment with no treatment, and 1 eye had undergone vitrectomy because of total retinal detachment. The AGV was removed due to plate exposure in one eye, but the IOP after AGV removal was within the normal range with tolerable medication.
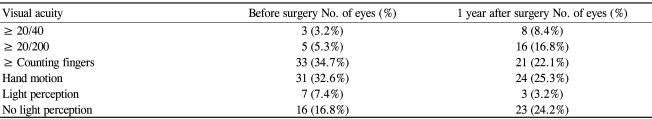
Visual acuity was within one line in 55 eyes (57.9%) but had worsened two lines or more in 17 eyes (17.9%) at 1 year after surgery. Seven eyes (7.4%) that had NVG and poor vision before surgery, ranging from counting fingers at 30 cm to light perception, lost light perception during the current study. In the present study, AGV implantations were performed in 16 eyes (16.8%) with no light perception. The purpose of the operation in these patients was to control pain and maintain the eyeball by lowering the IOP (Table 5).
In this study, 15 eyes (15.8%) were considered failures. 6 eyes (6.3%) had more than 21 mmHg with medication. An additional drainage device was implanted in 3 of these eyes (3.2%). Five of the 15 eyes (5.3%) failed because of hypotony and phthisis, and the implant was removed from the remaining eye (1.0%) due to plate exposure.
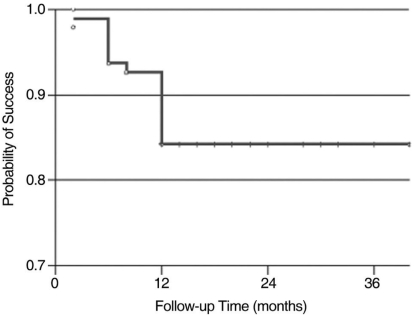
Based on our criteria for success, complete success was achieved in 28 eyes (29.5%), and qualified success in 80 eyes (84.2%) at 1 year. Kaplan-Meier survival analysis showed a cumulative probability of success of 84.2% after 12 months (Fig. 4).
Discussion
Early postoperative hypotony and its attendant complications, including choroidal detachment and suprachoroidal hemorrhage, still continue to present a clinical problem in valved glaucoma implants. The incidences of early postoperative hypotony in AGV implant surgery range from 8% to 13% using an intraocular pressure definition of <5 mmHg or 6 mmHg.5,8-12 In previous studies, suprachoroidal hemorrhage occurred in 1.7% to 4.7% of AGV implantations.5,8-12 This has been explained by valve failure, leakage around the tube, or a decrease in aqueous production.13,16 Of these possible causes of early postoperative hypotony, leakage around the tube is now prevented by corneal puncture using a 23-gauge needle.5
Kee13 used partial ligation of a silicone tube with 8-0 Vicryl in AGV, to restrict aqueous humor flow.13,14 He showed that the early postoperative hypotony and its surgical complications are less frequent if the AGV tube is partially ligated, but differences between the ligation and non-ligation groups were not statistically significant in his study, probably because of a small sample size.13
In the previous partial ligation study, the operator ligated both the tube and a stent (6-0 Prolene) with 8-0 Vicryl and then removed the stent. In the present study, the ligation of the AGV silicone tubes with 8-0 Vicryl was done in combination with the use of 3 strands of 8-0 Nylon as external stents. We did not remove the stents intraoperatively (complete ligation). We decided when to remove the stents according to the postoperative IOP. When IOPs failed to reduce postoperatively, stents were removed early to increase internal tube diameters. This method may have advantages compared to the previous partial ligation method in that IOP control is easier and more adjustable because the time of stent removal can be decided according to the postoperative state. The stents were simply removed with a forceps under a slit lamp because they remained exposed to the conjunctival surface. Moreover, stent removals during the immediate postoperative period were accompanied by rapid and sufficient IOP reductions, which suggest that enlargement of the tube lumen induces enhanced outflow and subsequent IOP reduction. The disadvantage may be irritation due to the external stents, however, this can be minimized by ensuring an adequate length of the 8-0 Nylon strands so as not to touch the cornea and removal within one month in most cases.
There were two reasons for using three strands of 8-0 Nylon as external stents rather than one large single strand. One was to reduce conjunctival irritation because one thick suture creates a greater sense of a foreign body than three thinner strands. The second reason is that they were originally intended to allow high postoperative IOP adjustment by removing them one by one. During the course of the study they were removed en bloc in most cases for fast IOP control; however, in two cases where stents were serially removed, additional stent removal resulted in additional IOP reduction, suggesting the advantage of multiple thin external stents.
We have to consider the reason for the occasional lack of a postoperative day one IOP spike despite the complete ligation of the tube. One possibility is that complete ligation was not performed in all cases because there might have been a minute space open inside the lumen of the ligated tube, or there is a possibility that there might be a flow of aqueous through the space between the tube and the corneoscleral ostium in the early postoperative period.
As a result of ligation and external stenting, 3 eyes (3.2%) showed an IOP of <5 mmHg on the first postoperative day. All hypotonic eyes recovered to the normal range within 2 weeks. A total of 6 eyes (6.3%) had a shallow anterior chamber on the first postoperative day in this study. We had no experience of flat or shallow anterior chambers that required surgical chamber reformation during the course of the study, and no suprachoroidal hemorrhage occurred. A choroidal effusion occurred in one eye with congenital glaucoma and an IOP of 6 mmHg on the first postoperative day, but this spontaneously regressed and did not require surgical intervention.
Molteno and Dempster14 reported a transient IOP elevation following a short-lived HP after single-plate Molteno implant surgery. This HP has been also reported for AGV implantation.4,9,10 The HP appeared approximately 4 weeks after surgery and lasted for at least 12 to 16 weeks. This phase is caused by histological changes in the bleb around an implant end-plate related to the formation of a layer of fibrous tissue in the bleb after diffuse edema and congestion disappears. The fibrotic capsule formed is resistant to aqueous flow, and thus results in an IOP elevation.13,16,17
In the current study, it was found that the HP appeared after an average of 2.7±1.5 weeks in 49 eyes (51.6%), the occurrence of the HP could not be ascertained in 25 eyes (26.3%) due to the use of medications. The mean highest IOP in those with a HP was 29.8±6.9 mmHg. The mean IOP profile also started to increase between 1 and 2 weeks after a fall in IOP on the first postoperative day. The mean IOP peaked from 2 weeks to 1 month postoperatively, and then stabilized over the ensuing months. Although the HP occurred earlier than in previous studies, the incidence of a HP and the mean highest IOP in those that developed a HP were similar to those reported for conventional AGV implant surgery. Tube ligation and external stent placement did not affect the HP, except for the time of occurrence. This is believed to be caused by an early IOP elevation due to the tube ligation.
In this study, the mean number of anti-glaucoma medications was larger at 1 month postoperatively (1.5±1.2) than has been reported previously (0.4 to 0.82), though the mean medication number at 1 year postoperatively was similar to those of other studies.9,10 An early increase in medication is also related to the early appearance of the HP.
The cumulative probability of success at 1 year was 84.2% in the present study, which compares favorably with the success rates reported by previous studies (70% to 87%),8,13-16,18,19 although some of the criteria used to measure success differ between studies.
Our study has the limitation that it is not a prospective, randomized, controlled trial. Despite its limitations, no other review has been conducted on the long-term follow-up data of a large number of patients that have undergone AGV implantation with tube ligation and external stents.
In conclusion, AGV implantation with tube ligation and external stents prevented hypotony-related early complications requiring surgical intervention. Moreover, the described method was found to result in a satisfactory IOP reduction in refractory glaucoma at 1 year postoperatively with a qualified success rate of 84.2%, comparable to those reported previously.
Notes
The contents in this paper were presented in the 94th Annual Meeting of the Korean Ophthalmological Society in Seoul on October 7, 2005.