Anomalous Scleral Insertion of Superior Oblique in Axenfeld-Rieger Syndrome
Article information
Abstract
Axenfeld-Rieger syndrome (ARS) is associated with ocular and systemic anomalies. PITX2 is known to be a major controlling gene in the pathogenesis of ARS and is associated with differentiation in both the neural crest and mesoderm during eye development.
A 4-year-old girl with bilateral ARS had 20 prism diopters (PD) of exotropia with 30PD of A- pattern deviation, more than 20PD of dissociated vertical deviation (DVD), and severe superior oblique overaction (SOOA). During surgery we observed that the SO inserted more posteriorly than normal.
We believe this finding is one of the abnormal manifestations of the development of the extraocular muscles in ARS.
We describe a patient with bilateral Axenfeld-Rieger syndrome (ARS) who had an anomalous, posterior-directed, scleral insertion of the superior oblique (SO) in both eyes. We think this finding is one of the abnormal manifestations of the development of the extraocular muscles in ARS.
Case report
The patient was a 4-year-old girl with bilateral ARS. On anterior segment examination, she had iris abnormalities of hypoplasia, corectopia, and sclerocornea prominent at the lateral limbus of both eyes (Fig. 1A, B). The patient also had a prominent posterior embryotoxin on gonioscopy. Intraocular pressure (IOP) was within the normal range. She had facial features of maxillary hypoplasia and dental hypoplasia. Her best corrected visual acuity was 6/60 in both eyes. Cycloplegic retinoscopy was -3.5 diopters (D) in the right eye and -4.0D in the left eye. Ocular alignment revealed 20 prism diopters (PD) of exotropia with 30PD of A-pattern deviation, more than 20PD of dissociated vertical deviation (DVD), and severe SO overaction (SOOA) (Fig. 2A). DVD was frequently manifested in the left eye and SOOA was also more severe in the left eye. After detaching the superior rectus (SR) during surgery, we observed that the SO inserted more posteriorly than would normally be expected. The distances between the medial insertion of the SO and that of the SR and between the lateral insertion of the SO and that of the SR were 16 mm and 11 mm in the right eye, respectively, and 15 mm and 11 mm in the left eye, respectively (Fig. 1C). Unfortunately, we lost the photograph of the left eye.

Photographs of the anterior segment showing corectopia, iris hypoplasia, and sclerocornea in the right (A) and left (B) eyes. The anomalous, posterior-directed, scleral insertion of the superior oblique muscle (arrowheads) and the stump of the insertion of the superior rectus (arrows) in the right eye (C).
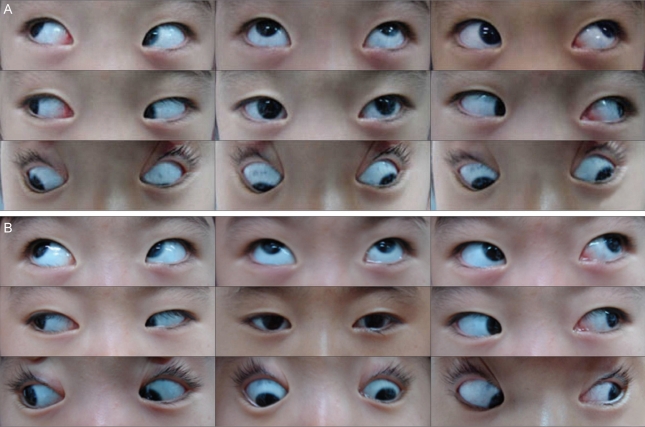
Preoperative photographs of eye motility demonstrating exotropia with A-pattern deviation and superior oblique overaction in both eyes (A). Postoperative photographs of eye motility (B).
The SO was composed of a fibrous band along its whole course under the Tenon tissue. There was no 'whitish, fanned-out fiber' temporal to the SR. The scleral insertions and the structures of the other rectus muscles were within normal limits for both eyes. We performed a 6 mm resection of the left medial rectus, an 8 mm recession of the SR, and a 4/5 posterior tenectomy of the SO in both eyes. Six months after surgery, the patient showed 6PD of exotropia in the primary position, 12PD of A-pattern deviation, DVD (especially in the left eye), and moderate SOOA (especially in the right eye) (Fig. 2B).
Discussion
ARS is a clinical entity exhibiting anterior segment dysgenesis of Schwalbe's line, iris hypoplasia, corectopia, iris strands to the iridocorneal angle, glaucoma, and sclerocornea.1 Non-ocular anomalies including a broad flat nose, maxillary hypoplasia, hypertelorism, dental hypoplasia, periumbilical skin folds, and heart defects are also manifested in ARS.2 All of the associated ocular and systemic anomalies are known to arise from the maldevelopment of the neural crest. To date, three chromosomal loci (4q25, 6p25, 13q14) and two genes (PITX2 and FOXC1) have been linked to ARS.3 In particular, PITX2 is known to encode a homeodomain transcription factor expressed in both the neural crest and mesoderm during eye development.4,5 PITX2 regulates the expression of other genes during embryonic development.6 Extraocular muscles are known to derive from the periocular mesoderm.4,6 We therefore think that the development of the extraocular muscles is influenced by PITX2, although the underlying genetic cascades have not been fully identified. Diehl et al demonstrated that the morphogenesis of all extraocular muscle bundles is highly correlated with PITX2 gene expression and that the SO and inferior oblique muscles were the most sensitive to PITX2 expression.4 Based on these observations, we hypothesize that PITX2 influences the development of both the anterior segment and extraocular muscles in ARS. To our knowledge, no study has reported anomalous extraocular muscle insertion in conjunction with ARS in patients with strabismus. We believe that our findings are beyond the normal anatomic variations.7 In conclusion, we think that the anomalous structure and insertion of the SO in our patient is an abnormal manifestation of the development of the extraocular muscles in ARS. Many patients with similar findings will need to be examined to elucidate the exact genetic mechanism behind this finding.