Intravitreal Bevacizumab for Treatment of Diabetic Macular Edema
Article information
Abstract
Purpose
To evaluate the effect of intravitreal bevacizumab on visual function and retinal thickness in patients with diabetic macular edema (DME).
Methods
Thirty eyes of twenty-eight patients (mean age, 57.9±13.8 years) with DME were included in this study. Complete ophthalmic examination, including determination of best-corrected visual acuity (BCVA), stereoscopic biomicroscopy, and retinal thickness measurement by optical coherence tomography (OCT), was done at baseline and at each follow-up visit. All patients were treated with a 0.05 mL intravitreal injection containing 1.25 mg of bevacizumab.
Results
All patients completed 3 months of follow-up with a mean follow-up period of 5.26±2.39 months. The mean BCVA at baseline was 0.73±0.36 logMAR, which significantly improved to 0.63±0.41 (p=0.02), 0.58±0.36 (p=0.003), and 0.61±0.40 logMAR (p=0.006) at 1 week, 1 month, and 3 months. Final BCVA analysis demonstrated that 15 eyes (50%) remained stable and 12 (40%) improved ≥2 lines on BCVA. The mean central retinal thickness was 498.96±123.99 µm at baseline and decreased to 359.06±105.97 (p<0.001), 334.40±121.76 (p<0.001), 421.40±192.76 µm (p=0.035) at 1 week, 1 month, and 3 months. No ocular toxicity or adverse effects were observed.
Conclusions
Intravitreal bevacizumab injection resulted in significant improvement in BCVA and central retinal thickness as early as 1 week after injection in patients with DME, and this beneficial effect persisted for up to 3 months. However, the slight reduction in this improvement at 3 months suggests that repeated bevacizumab injections might be necessary. To evaluate the long-term safety and efficacy, further prospective randomized controlled clinical trials will be needed.
Diabetic macular edema (DME) is the leading cause of visual loss in patients with diabetes mellitus, and it frequently leads to irreversible changes in visual acuity.1 DME is caused by excessive vascular permeability, which leads to leakage of fluid and plasma constituents, such as lipoproteins, into the retina. This then causes retinal thickening. The Early Treatment Diabetic Retinopathy Study (ETDRS) showed that focal laser photocoagulation is beneficial in the treatment of clinically significant macular edema, reducing the rate of moderate visual loss by 50%.2 However, only 3% of patients improved by ≥3 lines of vision by the end of the study. Intravitreal triamcinolone acetonide (IVTA) injection has proven effective in improving vision and reducing macular thickness in DME, both as an initial treatment and as a second line therapy after unsuccessful laser therapy.3,4 However, its effect is temporary, and a number of side effects have been reported.5,6 Consequently, its therapeutic value remains unclear.
Vascular endothelial growth factor (VEGF) has been implicated as an important factor in the breakdown of the blood-retina barrier, with increased vascular permeability resulting in retinal edema in diabetic patients through affecting endothelial tight junction proteins.7 While the normal human retina contains VEGF, hypoxia stimulates the secretion of VEGF from retinal pigment epithelial cells.8,9 VEGF levels are significantly elevated in eyes with DME.10,11 In addition, VEGF concentrations are significantly higher in eyes with extensive macular leakage when compared to eyes with minimal leakage.11 Therefore anti-VEGF treatments have been proposed as an alternative adjunctive treatment for DME.12 Bevacizumab (Avastin, Genentech Inc., San Francisco, CA) is a complete full-length humanized antibody that binds to all subtypes of VEGF; it has been used successfully as a systemic drug in tumor therapy.13 Recent studies have demonstrated the usefulness of intravitreal injections of bevacizumab in the reduction of macular edema secondary to central retinal vein occlusion, vascular permeability, fibrovascular proliferation in retinal neovascularization secondary to proliferative diabetic retinopathy (PDR), and choroidal neovascularization secondary to age-related macular degeneration (AMD).14-17
The purpose of this retrospective study was to evaluate the effect of intravitreal bevacizumab on visual function and retinal thickness in patients with DME.
Materials and Methods
The present study was designed as a retrospective, consecutive case series study of eyes with DME treated with off-label intravitreal bevacizumab between March 2007 and February 2008. We reviewed the clinical records of 28 consecutive patients (30 eyes) with DME treated with at least one intravitreal injection of 1.25 mg of bevacizumab. Because there are no evidence-based indications for the treatment of DME with bevacizumab, we included a wide range of patients with diffuse, clinically significant DME who did not respond to other treatments such as photocoagulation, intravitreal triamcinolone injection, or pars plana vitrectomy. The inclusion criteria for the study eye included (1) best-corrected visual acuity (BCVA) ≤20/40, (2) clinically definite retinal thickening due to DME involving the center of the macula, (3) optical coherence tomography (OCT) central retinal thickness ≥275 µm, and (4) no history of treatment for DME within the prior 3 months. Exclusion criteria included macular edema due to a cause other than diabetes, other ocular condition that might affect macular edema or alter visual acuity, and evidence of external ocular infection.
Each patient underwent a complete eye examination, including determination of BCVA, slit-lamp examination, intraocular pressure (IOP) measurement, stereoscopic biomicroscopy of the retina using a 90-diopter lens, and retinal thickness measurement by OCT (Stratus OCT model 3000; Carl Zeiss Meditec Inc., San Leandro, CA), at baseline and at each visit. Patients were examined at 1 week and 1 month after injection, and then at 1- or 2-month intervals at the discretion of the investigator. OCT images were obtained by fast macular thickness map scan (6-radial line pattern), and the central retinal thickness was measured using retinal map analysis for the calculation of average thickness at the center point. Each patient's BCVA was transferred from his or her records and converted to a logarithm of the minimum angle of resolution (logMAR) scale for analysis.
Topical anesthesia was induced by applying proparacaine (0.5%) eye drops before injection. The conjunctiva bulbi and the fornices were repeatedly rinsed with povidone-iodine, which was also applied to the eyelid margins and the lashes to avoid expression of the meibomian glands. After application of a sterile drape and subconjunctival injection of anesthesia (2% lidocaine containing 1:100,000 epinephrine), a 30-gauge needle on a 1 cm3 syringe was used to inject bevacizumab intravitreally through the pars plana 3.5 to 4.0 mm posterior to the limbus, at a dose of 1.25 mg in 0.05 mL. The needle was carefully removed using a sterile cotton applicator to prevent reflux. After injection, antibiotic eye drops were applied four times per day for 3 days.
All patients provided written informed consent, and they were informed of the off-label use of the drug and its potential risks and benefits, as well as the likelihood that additional treatments might be required. The paired t-test was used for comparison of preoperative and postoperative BCVA and central retinal thickness. The multiple regression analysis was performed to evaluate the associated factors influencing treatment success, and Mann-Whitney U test was used to compare response to treatment between nonvitrectomized eyes and previously vitrectomized eyes. For all statistical tests, a p value <0.05 was considered statistically significant. The data were analyzed using statistical software (SPSS, version 12.0, SPSS Inc, Chicago, Illinois, USA).
Results
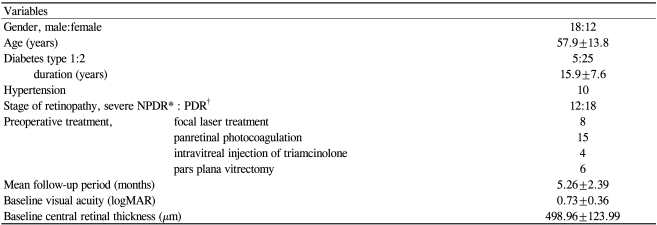
Thirty eyes (28 patients) with a minimum of 3 months follow-up were included for analysis. The mean patient age was 57.9±13.8 years, and 60% were male (18 men, 12 women). All patients completed 3 months of follow-up, with a mean follow-up period of 5.26±2.39 months (range, 3-11 months). Type 2 diabetes was present in 83.3% of patients, and type 1 diabetes was present in 16.7% of patients. Eighteen eyes (60%) exhibited PDR, and twelve exhibited severe nonproliferative diabetic retinopathy (NPDR). Twenty-five eyes (83.3%) had received at least one alternative therapy before intravitreal bevacizumab injection. Focal laser therapy had been applied once in 4 eyes and more than twice in 4 eyes. Full scatter panretinal laser therapy had been performed on 15 eyes (50%), and 6 eyes (20%) had undergone pars plana vitrectomy. Previous intravitreal injection of triamcinolone acetonide had been performed on 4 eyes at least 3 months before undergoing intravitreal bevacizumab injection. Additional baseline characteristics by treatment group are depicted in Table 1.
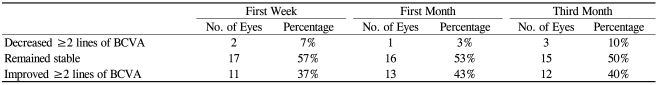
Improvements in visual acuity were noted from 1 week after intravitreal bevacizumab injection, and these statistically significant changes continued throughout the 3-month follow-up visit (Fig. 1). At baseline, the mean BCVA was 0.73±0.36 logMAR. This improved significantly to 0.63±0.41 (p=0.02), 0.58±0.36 (p=0.003), and 0.61±0.40 logMAR (p=0.006) at 1 week, 1 month, and 3 months, respectively. At 3-month follow-up, the BCVA was slightly decreased, but there was no significant difference in the mean BCVA between the 1- and 3-month follow-up visits (p=0.536). The visual acuity results are summarized in Figure 2. Final BCVA analysis by subgroup demonstrated that 15 (50%) of 30 eyes remained stable, 12 (40%) improved ≥2 lines on BCVA, and 3 (10%) deteriorated ≥2 lines on BCVA (Table 2).

Changes in best-corrected visual acuity (BCVA) and central retinal thickness measured by optical coherence tomography (OCT) after intravitreal bevacizumab injection.
Mean central retinal thickness was 498.96±123.99 µm (range, 275-733 µm) at baseline by OCT. At 1 week postoperatively, the mean central retinal thickness measurement decreased to 359.06±105.97 µm (p<0.001), and this significant improvement of retinal thickening continued through the 1- and 3-month follow-up visits (p<0.001 and p=0.035, respectively) (Fig. 1). However, at 3-month follow-up, mean central retinal thickness significantly increased to 421.40±192.76 µm, compared with 1-month follow-up (334.40±121.76 µm, p=0.044). Figure 3 summarizes OCT-measured retinal thickness results.
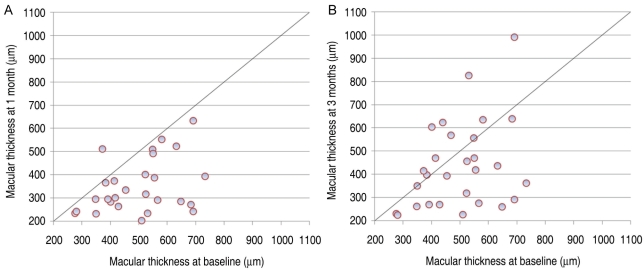
Development of central retinal thickness measured by optical coherence tomography after 1 month (A) and 3 months (B) of follow-up.
Changes in visual acuity and changes in central retinal thickness did not vary substantially according to subject age (p=0.54 and p=0.95, respectively), diabetic retinopathy severity (p=0.88 and p=0.14), previous focal laser treatment (p=0.09 and p=0.76), previous panretinal photocoagulation (p=0.31 and p=0.93), or previous IVTA injection (p=0.79 and p=0.83). There was a suggestion of greater effect on visual acuity in eyes that had not undergone pars plana vitrectomy compared with previously vitrectomized eyes (p=0.025). The same effect was not observed concerning central retinal thickness (p=0.98). Eyes with thicker retinas at baseline experienced a greater absolute and relative reduction in central retinal thickness at 1 month (p=0.001 and p=0.031, respectively), but changes in visual acuity did not differ according to baseline visual acuity or baseline central retinal thickness (p=0.06 and p=0.63, respectively).
There were no cases of endophthalmitis, uveitis, IOP increase, or severe decrease in vision immediately after injection. At 3 months, no ocular or systemic adverse events were reported, including thromboembolic events (cerebrovascular accidents, transient ischemic attacks, myocardial infarctions, or peripheral vascular disease).
Discussion
DME is a manifestation of diabetic retinopathy that produces severe visual impairment. Although several treatment modalities are under investigation, the only demonstrated means to reduce the risk of vision loss from DME are laser photocoagulation, as demonstrated by the ETDRS2; intensive glycemic control, as demonstrated by the Diabetes Control and Complications Trial and the United Kingdom Prospective Diabetes Study; and blood pressure control, as demonstrated by the United Kingdom Prospective Diabetes Study.18,19 However, there has been interest in other treatment modalities, such as pharmacologic therapy with oral protein kinase C inhibitors and the use of intravitreal corticosteroids, because most laser-treated DME eyes do not exhibit satisfactory improvements in VA.20,21 Antibodies targeted to VEGF have also generated considerable interest and are being investigated.
VEGF is an endothelial cell-specific mitogen and angiogenic inducer in a variety of in vitro and in vivo models.22 It is upregulated by hypoxia, and it plays a role in DME and contributes to the excessive vascular permeability that leads to macular edema in diabetic patients. Bevacizumab is a full-length humanized monoclonal antibody that binds and inhibits all biologically active isoforms of VEGF. Although preclinical experimental data from primates suggested that the full-length antibody might not penetrate the internal limiting membrane of the retina, recent studies have shown full-thickness penetration of the retina within 24 hours.23,24 To our knowledge, all clinical and experimental studies presented thus far have not noted drug-related toxic effects in any retinal structure.14-17,25-30 Intravitreal injection of bevacizumab appears to have good efficacy in the treatment of wet AMD as a new treatment option. Injection of bevacizumab into the vitreous cavity, as is presently done mostly for patients with AMD, is based on the results of clinical reports clearly indicating an increase in visual acuity and a decrease in retinal thickness.15,16,27 In addition, other VEGF inhibitors, such as pegaptanib sodium (Macugen) - which binds to one VEGF isoform - have also been successfully used to treat DME in a published randomized, controlled, double-masked phase II multicenter trial. Subjects treated with pegaptanib had better visual acuity outcomes, were more likely to have reduction in central retinal thickness, and were less likely to need additional photocoagulation therapy at follow-up.12 In light of this information, intravitreal bevacizumab injection is expected to have good efficacy in the treatment of DME.
Recently, Haritoglou et al.28 published a prospective, noncomparative case series of patients with DME treated with 1.25 mg bevacizumab. There was a significant reduction in macular thickness at 2 weeks (p=0.002) and although mean visual acuity improved significantly at 6 weeks (p=0.02), this was not sustained at 12 weeks. In the present investigation, however, we found that significant improvement in both visual acuity and retinal thickness was achieved soon after intravitreal bevacizumab injection, and the beneficial effects lasted for 3 months. The mean BCVA improved from 0.73±0.36 logMAR at baseline to 0.63±0.41 logMAR at 1-week follow-up (p=0.02), and the mean central retinal thickness as measured by OCT also decreased significantly from 498.96±123.99 µm at baseline to 359.06±105.97 µm at 1-week follow-up (p<0.001). At 1 month after the injection of bevacizumab, this beneficial effect on BCVA and central retinal thickness appeared to be most prominent in the current study. Thirteen (43%) of 30 eyes showed an improvement in BCVA by 2 or more lines, and only 1 eye (3%) decreased ≥2 lines on BCVA at 1-month follow-up. In addition, the central retinal thickness showed a considerable reduction (33%): from 498.96±123.99 µm at baseline to 334.40±121.76 µm at 1 month. Although the duration of action of intravitreal bevacizumab is unknown, recent electrophysiologic and retinal penetration studies have reported that full thickness retinal penetration is present at 24 hours.24 This may explain the earlier clinical effects of intravitreal bevacizumab observed in the current study.
A recent report from the Pan-American Collaborative Retina Study Group showed a significant decrease of DME one month after intravitreal injection of bevacizumab, and those decreased levels were kept for up to six months.29 In our study, the reduction of central retinal thickness and the improvement of BCVA were observed at 1 week, and these results were maintained for up to 3 months. However, at 3-month follow-up, a slight decrease in visual acuity and an increase in retinal thickness were observed as compared with the 1-month follow-up. The central retinal thickness increased significantly from 334.40±121.76 µm at 1 month to 421.40±192.76 µm at 3 months (p=0.044). This slight reduction in the improvement of visual acuity and central retinal thickness at 3-month follow-up suggests that repeated intravitreal bevacizumab injections may be necessary within 3 months to maintain a beneficial effect. In this study, 6 (20%) of 30 eyes received the second injection at a mean of 3.8 months. Re-treatment was done only in cases of BCVA deterioration and increased macular edema on OCT. It is possible that a different dosing schedule, such as a series of injections every 3 months for an extended period, may be superior to the method used in this study. However, we chose to re-treat the recurrent cases only, because data concerning toxicity and duration of action of intravitreal bevacizumab were not sufficient at the beginning of the study.
The breakdown of endothelial tight junctions and loss of the blood-retina barrier that lead to DME can be associated with both nonproliferative diabetic retinopathy and PDR. The present study demonstrates a comparable population of PDR and NPDR patients with macular edema. The results of the present study indicate that intravitreal bevacizumab injections may have a beneficial effect on retinal thickness and visual acuity, independent of the type of diabetic retinopathy. In addition, previous treatments, such as focal laser treatment, panretinal photocoagulation, or IVTA injection, did not influence the results of the study, except in the case of previous vitrectomy. Previously vitrectomized eyes showed no increase in visual acuity, and this finding is consistent with a previous study that found there was no change in BCVA or foveal thickness after intravitreal bevacizumab injection for DME in previously vitrectomized eyes.30 This outcome may be attributable to rapid clearance of intravitreal bevacizumab and insufficient sustained therapeutic levels in vitrectomized eyes. Further studies are warranted due to the relatively small number of participants in this study.
In conclusion, intravitreal bevacizumab injection appears to result in significant improvement in BCVA and reduction in central retinal thickness as early as 1 week after injection, and this beneficial effect was shown to persist for up to 3 months. However, the slight reduction in improvement in visual acuity and central retinal thickness at 3-month follow-up suggests that repeated bevacizumab injections might be necessary within 3 months to maintain its effect, as the drug is well tolerated and there are no safety concerns. To evaluate the long-term safety and efficacy of this new treatment, further prospective randomized controlled clinical trials will be needed, with scheduled re-injection and longer follow-up.
Notes
The author has no proprietary interest in any of the materials or equipment mentioned in this study.