Transforming growth factor-β Does Not Induce Endothelin-1 Secretion in Primary Cultured Human Tenon's Fibroblasts
Article information
Dear Editor
Subconjunctival fibrosis is closely related to scarring after glaucoma filtering surgery, and transforming growth factor-β (TGF-β) is known to play a crucial role in this scarring process. It induces the transformation of fibroblasts to myofibloblasts and the production of extracellular matrix (ECM) in the Tenon's capsule. Endothelin-1 (ET-1) is thought to be secreted by fibroblasts and contributes to fibrosis in such stressed conditions. However, evidence regarding autocrine production of ET-1 from TGF-β stimulated human Tenon's fibroblasts are lacking. In the present study, the authors evaluated the effects of TGF-β1 on ET-1 expression from primary cultured human Tenon's fibroblasts. Our results demonstrate that TGF-β1 seemingly has no influence on the synthesis of ET-1 in human Tenon's fibroblasts. Even though fibroblasts usually are not a major source of ET-1, these different cellular responses may implicate the characteristics of Tenon's fibroblasts as being different to fibroblasts from other tissue. Studies concerning other intracellular signaling pathways associated with pro- and anti- fibrosis are also needed to confirm the similarities of fibroblasts from human Tenon's capsule with that from other organs.
For several decades, the mechanism associated with subconjunctival fibrosis has been a matter of great concern to glaucoma specialists because scarring affects the outcome after glaucoma filtering surgery. Transforming growth factor-β (TGF-β) is known to play an important role in this scarring process, and many in vitro and in vivo studies reveal topical anti-TGF-β antibodies blocking myofibroblastic transformation of fibroblasts and reducing extracellular matrix (ECM) production in Tenon's capsule.1-5
Fibroblast is a type of cell that synthesizes and maintains the ECM of various tissues. It plays an important function in wound healing but can also bring about over-fibrosis. Accordingly, the fibrotic and anti-fibrotic mechanisms have been studied widely in fibroblasts from various organs including the lung, kidney, dermis, etc. Endothelin-1 (ET-1) is a vasoconstricting peptide produced primarily in the endothelium and seems to play a key role in vascular homeostasis. However, under stressed conditions, it can be secreted by other types of cells like cardiac myocytes and even fibroblasts. Regarding current thought on the association between the ET-1 and TGF-β, there are two contrary opinions; the TGF-β stimulates the autocrine production of ET-1 in pulmonary and placental fibroblasts but not in dermal fibroblasts.6-10 Because ET-1 binds to the cell surface receptors of fibroblasts and increases cellular proliferation and ECM production, TGF-β is thought to induce autocrine production of ET-1 in human Tenon's fibroblasts. So, in the present study, the authors evaluated the effects of TGF-β1 on ET-1 expression from primary cultured human Tenon's fibroblasts.
After obtaining approval from the Institutional Review Board, small human Tenon's capsule specimens were obtained during strabismus surgery in compliance with the tenets of the Declaration of Helsinki. Primary cultured human Tenon's fibroblasts were stimulated with 5 ng/mL of recombinant human TGF-β1 (R&D System, Inc., Minneapolis, MN) up to 48 hours (at 0, 1, 3, 6, 12, 24, and 48 hours) after the initiation of TGF-β1 treatment. Whole cellular proteins were extracted and Western immunoblots was performed with primary antibodies as indicated. Antibodies against ET-1, collagen type I, fibronectin, and β-actin were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Intensity of each band was calculated as compared with that of 0 hr time point, and all procedures were performed in triplicate.
Representative Western immunoblots are shown at Figure 1 and the intensity data are demonstrated at Figure 2. As compared with control (at 0 hr), the synthesis of collagen type I and fibronectin increased with time by more than 3 times. This increase was more apparent after 12 hours. However, a band that represents ET-1 was hardly detected on all samples and it did not show any significant change. Beta-actin, an internal control, also was not changed.
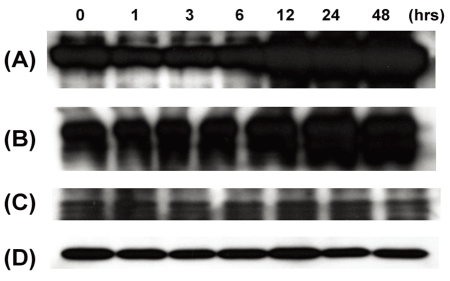
Representative Western immunoblots. (A) collagen type I, (B) fibronectin, (C) endothelin-1, and (D) beta-actin.
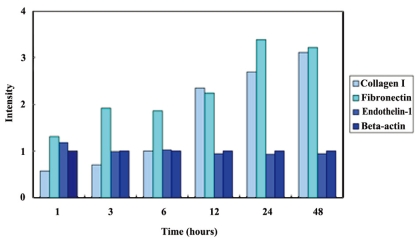
Intensity of bands in Western immunoblots. Intensity of each band was calculated as compared with that of 0 hr time point.
Our results demonstrate that TGF-β1 has no influence on the synthesis of ET-1 in human Tenon's fibroblasts and the fibroblasts of human Tenon's capsule seem to have no autocrine function for the ET-1. Even though fibroblasts usually are not a major source of ET-1 even in other extraocular tissues, these different cellular responses may implicate that characteristics of Tenon's fibroblasts are different from fibroblasts of other tissue. Further study about their response to exogenous ET-1 is required and other intracellular signaling pathways associated with pro- and anti- fibrotic courses are also needed to be confirmed directly. Understanding the precise mechanisms of the fibrotic process may give promise to more successful glaucoma filtering surgery.
Acknowledgements
This work was supported by a Faculty Research Grant of Yonsei University College of Medicine 2006 (6-2006-0061).