Dear Editor
Alpha-synuclein is a widespread neuronal presynaptic protein found predominantly in the central nervous system, and it is an element of the neuropathological features of several neurodegenerative disorders including the dementia with Lewy bodies and the Parkinson's disease. At the extra-neural tissue, the normal distribution and functions of α-synuclein remain especially elusive. Though there was one report that described the absence of α-synuclein in human cornea using the Western blots, we had access to data from immunohistochemical staining with fluorescence indicating high immunoreactivity of α-synuclein in human corneal epithelium and endothelium. Our results are consistent with the expression of α-synuclein in the normal human cornea. The presence of α-synuclein in normal human cornea suggests that this molecule is widely distributed and functions in mammalian extra-neural tissue.
Synucleins are small proteins (112-140 amino acids) that are transported axonally after their synthesis in the cell body. Alpha-synuclein, a member of synuclein family, is a widespread neuronal presynaptic protein found predominantly in the central nervous system and with lower expression in other tissues.1-3 It has been implicated specifically in several neurodegenerative diseases, and it is an element of the neuropathological features of several neurodegenerative disorders including the dementia with Lewy bodies and the Parkinson's disease.4-7 It is largely accepted that intraneural aggregates of α-synuclein are neurotoxic, though there are some evidences that they may have some protective role.8-10
Even though there are considerable informations pointing to the role of α-synuclein in the different human diseases, its normal distributions and functions especially at the extra-neural tissue remain elusive. In the ocular tissue, Surguchov et al.3 reported the presence of α-synuclein in the human retina and optic nerve. Recently, we had access to data from immunohistochemical staining with fluorescence indicating high immunoreactivity of α-synuclein in the human corneal epithelium and endothelium. As such, we were surprised by the study of Surguchov et al.3 who reported the absence of α-synuclein in the human corneal tissue using the Western blots.
We used immunohistochemical staining with fluorescence to confirm that the α-synuclein was indeed expressed in the human cornea. We certify that all applicable institutional and governmental regulations concerning the ethical use of human tissues were followed during this research. And all procedures followed the guidelines of the declaration of Helsinki.
Three normal human eyeballs (age 36 to 50 years) donated for keratoplasty were used. After removal of corneal buttons for keratoplasty, the remained tissue containing the peripheral cornea was fixed with 4% p-formaldehyde and subsequently embedded in paraffin. Each specimen was serially sectioned into 4-µm sections and mounted on glass slides. After serial paraffin sections were deparaffinized in xylenes and rehydrated with a graded concentration of ethanol, immunohistochemical staining was performed using the ABC Elite system (Vector Laboratories, Burlinghame, CA) according to the manufacturer's instructions. Briefly, the sections were incubated with horse serum for 30 minutes. After several washing steps, the sections were incubated with the mouse monoclonal IgG (1:500 dilution) specific for human α-synuclein (Santa Cruz Biotech, Santa Cruz, CA) for 12 hr at 4℃. Binding of the primary antibody was detected with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (1:200 dilution, Sigma, St. Louis, MO) after 1 hour of incubation at room temperature. The slides were examined with a fluorescent microscope.
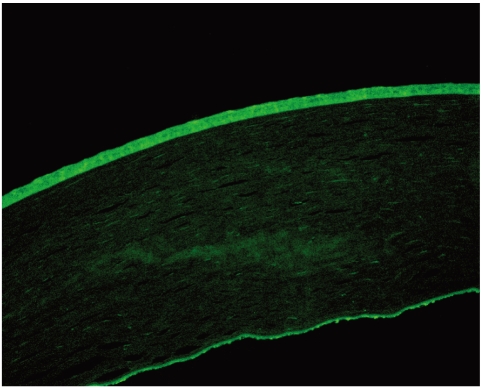
All three of the donors had normal eyes without any ocular pathology, and they had no history nor family history of any neurodegenerative diseases. In order to investigate the expression of α-synuclein in normal cornea, we probed three corneal tissues with the antibody against α-synuclein. The staining of the specimens was demonstrated with fluorescent microscopy. Alpha-synuclein was highly expressed in corneal epithelium and endothelium (Fig. 1). However, it was not stained in corneal stroma. Under identical conditions, no fluorescence was obtained with sections not exposed to the primary antibody and solely incubated with the FITC-conjugated secondary antibody.
Our results are consistent with the expression of α-synuclein in the normal human cornea. We cannot account for the discrepancy between our results and those of Surguchov et al.3 However, we suspect that their method of Western blotting of the whole corneal lysate may have resulted in a less sensitive detection.
Even though we could not explain the exact role of the α-synuclein in normal human cornea, the presence of α-synuclein in normal human cornea suggests that this molecule is widely distributed and functions in mammalian extra-neural tissue.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print






