 |
 |
| Korean J Ophthalmol > Volume 20(2); 2006 > Article |
Abstract
Purpose
To evaluate the efficacy and safety of angle-supported phakic anterior chamber intraocular lenses in amblyopic adult eyes with very high myopia.
Methods
We evaluated 12 eyes in nine patients with very high myopic amblyopia who received angle-supported phakic intraocular lenses (Phakic 6H®) and followed them for more than six months. Uncorrected visual acuity (UCVA), best-corrected visual acuity (BCVA), and complications were evaluated. A satisfaction score was rated by patients using a 5-point (1~5) numeric scale.
Results
The mean age of patients was 37.3┬▒9.4 years, ranging from 29 to 59 years old. The preoperative mean refraction (spherical equivalent, SE) was -20.10┬▒5.41 diopters (D). The postoperative mean refraction (SE) was -1.75┬▒0.76 D at six months. The postoperative BCVA improved an average 3.92┬▒1.24 lines over preoperative values, and mean endothelial cell loss was 8.9% at six months. Development of cataracts, glaucoma, and pupil abnormalities were not demonstrated in any case during the study. The patients were all very satisfied, as the average satisfaction score was 4.3.
Conclusions
This study indicates that angle-supported phakic anterior chamber intraocular lens implantation may be an effective surgical alternative for the correction of amblyopic adult eyes with very high myopia. However, long-term evaluation is necessary to assess possible complications and long-term safety.
The surgical correction of very high myopia currently includes clear lens extraction,1,2 anterior and posterior chamber intraocular lens (IOL) implantation,3,4 and keratorefractive surgeries such as photorefractive keratectomy (PRK), laser in situ keratomileusis (LASIK), and radial keratotomy.5,6 However, clear lens extraction is associated with increased risk of retinal detachment, cystoid macular edema, endothelial cell loss, and endophthalmitis.2,7 Keratorefractive surgeries are associated with iatrogenic keratectasia, significant optical aberrations, regression, poor quality of vision, and poor predictability when used to correct high myopia.5,8-11
The implantation of phakic IOLs is an emerging technology in the treatment of high ametropia, since it avoids both the unpredictability of PRK and LASIK and the loss of accommodation of clear lens extraction.12 Phakic IOLs have significant advantages such as reversibility, immediate correction, stability, and relative simplicity.12-14 However, long-term studies are necessary to assess potential risks to the corneal endothelium, anterior uvea, and crystalline lens.3,7
The purpose of this study was to evaluate the efficacy, stability, and safety of angle-supported phakic anterior chamber intraocular lenses (PAC IOLs) in amblyopic adult eyes with very high myopia.
Angle-supported phakic IOLs (Phakic 6H®, Ophthalmic Innovations International Inc., USA) were implanted in adult amblyopic eyes with very high myopia between April 2001 and August 2003 by the same surgeon (K.H.S.). Inclusion criteria were: age of 20 years or older, myopia of -12.0D or higher in spherical equivalent, a BCVA of 20/70 or worse, anterior chamber depth (ACD) of at least 2.8 mm, endothelial cell count of at least 2,500 cells/mm2, and a wide-open angle (20~40 degrees) by the Shaffer's gonioscopic classification.15 Exclusion criteria included glaucoma, cataract, keratoconus, history of uveitis, corneal dystrophy, previous ocular surgery, and systemic diseases such as diabetes or hypertension, autoimmune diseases, and connective tissue diseases.
A preoperative ocular examination was conducted on each patient to evaluate distance UCVA and BCVA using a Snellen chart, manifest and cycloplegic refractions, Goldmann applanation tonometry, keratometry, corneal topography, slit lamp and fundus, gonioscopy, ultrasound pachymetry, white-to-white (WTW) limbal dimension, endothelial cell count (Cellcheck CC-7000, Konan), anterior chamber depth estimation by ultrasound A-scan, and pupil diameter.
All visual acuities were measured using letters on the Snellen chart and recorded in a standardized fashion, in which each letter read on a complete line was noted accordingly. Preoperative BCVA, postoperative UCVA, and postoperative BCVA were recorded in logMAR units; a change of 0.1 unit corresponded to a line of vision.
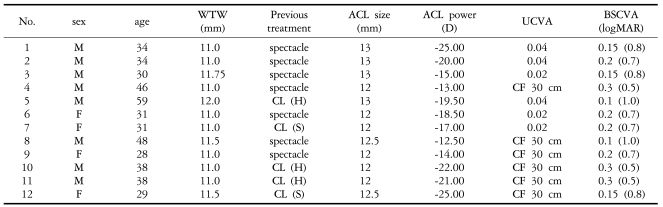
Lens-power calculations were performed with formulae developed by Ophthalmic Innovations International Inc. The overall diameter of the phakic IOL to be implanted was determined by measuring the WTW diameter with a caliper and then adding 1 mm. The target postoperative mean refraction (SE) was emmetropia.
Preoperative preparations included administration of 2% pilocarpine drops to the operative eye at 15-minute intervals and 15% mannitol 300 cc intravenously one hour prior to surgery. Nadbath akinesia and retrobulbar anesthesia with 2% lidocaine were used. One 1-mm paracentesis was made at the 3 o'clock position. A small amount of viscoelastic agent (Healon®, Pharmacia, Sweden) was injected to maintain a stable anterior chamber throughout the surgical procedure. A 7-mm valved limbal incision was created between the 10 and 2 o'clock positions with a bevel-up crescent knife. A 7-mm lens glide was introduced into the anterior chamber down to the 6 o'clock position, taking care to avoid contact with the iris, endothelium, and crystalline lens. The viscoelastic agent was again injected over the glide sheet. The phakic IOL was then introduced toward the 6 o'clock position and the glide was withdrawn. The phakic IOL was rotated to the meridian in which the pupil was best centered. After the lens had been placed in the eye, its stability was tested by pressing on the limbus in the meridian of the long axis of the lens to observe any unexpected movements or displacements of the lens. A peripheral iridectomy was performed using Vannas scissors. The incision site was closed with 10-0 nylon-interrupted sutures. Before the knot was tied, irrigation of the anterior chamber with a balanced salt solution was performed to remove any remaining viscoelastic agent. The suture was then knotted. Topical ofloxacin ointment was given at the end of surgery and the surgical site patched for 24 hours.
Postoperatively, 1% prednisone acetate (Pred Forte®, Allergan, USA) and 0.3% Ofloxacin (Tarivid®, Santen, Japan) were prescribed. Follow-up examinations were made at one day, one week and one, two, three, and six months following surgery. ANOVA was used to assess the significance of differences among subjects between repeated measurements.
The patients' satisfaction of vision was evaluated at six months postoperatively using a questionnaire. Patients were asked to subjectively evaluate their level of visual difficulty on a 5-point scale in five situations: distance vision, near vision, night halo and glare, improvement of working efficiency, and overall satisfaction. Response options were: 1=major trouble; 2=a great deal of trouble; 3=moderately satisfied; 4=satisfied; 5=very satisfied.
A total of 12 eyes in nine consecutive patients were recruited to the study, with six males and three females being followed for more than six months. The mean patient age was 37.3 years (ranging from 29 to 59 years). The preoperative mean UCVA was 0.01 (ranging from CF 30 cm to 0.04), and the mean BCVA was 0.18 (ranging from 0.1 to 0.3). The preoperative mean refraction (SE) was -20.10┬▒5.41D. The mean WTW diameter was 11.2┬▒0.4 mm (ranging from 11 to 12 mm), the mean overall diameter of the implanted phakic IOLs was 12.4┬▒0.5 mm (ranging from 12 to 13 mm), and the mean power of phakic IOLs was -18.54┬▒4.34D (ranging from -12.5 to -25.0D) (Table 1).
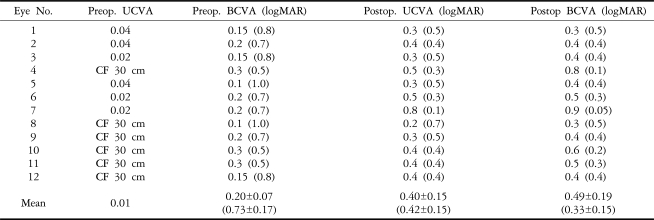
The postoperative mean SE was -1.74┬▒0.92D (ranging from -0.625 to -2.375D) at one week and -1.75┬▒0.76D (ranging from -1.25 to -2.00D) at six months (Table 2). There was no significant change in postoperative SE refraction at one week and one, two, three, and six months (p=0.83) (Fig. 1).
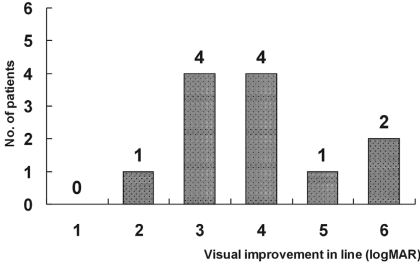
Mean postoperative UCVA was 0.24 (ranging from 0.1 to 0.6) at one week, 0.34 (ranging from 0.2 to 0.8) at one month and 0.40 (ranging from 0.2 to 0.8) at six months. Mean postoperative BCVA was 0.39 (ranging from 0.2 to 0.7) at one week, 0.44 (ranging from 0.2 to 0.8) at one month and 0.49 (ranging from 0.3 to 0.9) at six months (Table 3). The postoperative BCVA improved an average of 3.92┬▒1.24 lines (logMAR) compared with the preoperative values at six months (Fig. 2).
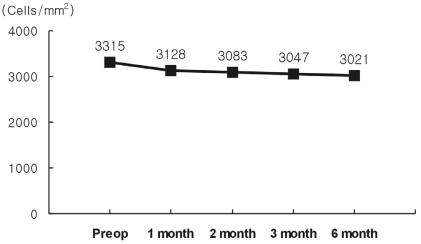
The preoperative mean endothelial cell density was 3315┬▒31 cells/mm2. After surgery, this decreased to 3128┬▒512 cells/mm2 at one month, 3021┬▒609 cells/mm2 at six months. The percentage of endothelial cell loss was 8.9% at six months (Fig. 3). The preoperative mean IOP was 14.5┬▒1.83 mmHg. After surgery, this increased to 17.1┬▒1.85 mmHg at one week, and 15.1┬▒2.01 mmHg at six months. None of the patients required glaucoma therapy to control IOP elevation continuously. The preoperative mean anterior chamber depth (ACD) was 3.40┬▒0.24 mm. At six months, the mean ACD was 3.00┬▒0.33 mm (a 4.1% decrease).
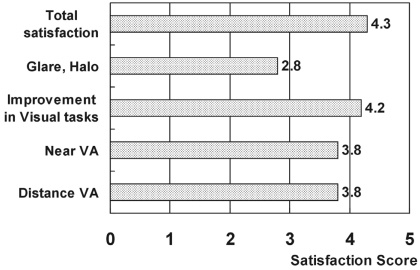
Development of complications such as cataracts, glaucoma, chronic uveitis, retinal detachment, endophthalmitis, IOL dislocation, and pupil abnormality were not demonstrated in any case during the study. The patients were all very satisfied and the average satisfaction score was 4.3 (Fig. 4).
Refractive correction of very high myopia is a difficult clinical problem. At this time, there is no satisfactory surgical procedure for the correction of very high myopia. The upper limit of correction that can be achieved with radial keratotomy is generally thought to be -8.0D.16 Although no precise limits have been determined, most surgeons do not perform LASIK in eyes with myopia higher than -12.0D.17 PRK has generated high expectations for the correction of low and moderate myopia. However, keratorefractive surgeries for the correction of very high myopia can be problematic because of limited predictability and the potential for irreversibility, regression, and corneal haze.6,18 Clear lens extraction followed by implantation of low-power IOLs have not gained wide acceptance due to complications such as retinal detachment, which can lead to early loss of accommodation in relatively young patients.2,4,6
Phakic IOLs are increasingly popular with refractive surgeons because of refractive predictability and stability, and because they do not alter accommodation.19 All of the above-mentioned reasons support the use of PAC IOLs for the correction of refractive errors. However, there are potentially important risks that need to be clarified before the procedure is put into general use.
One important risk is that angle-supported phakic IOLs may induce damage to the anterior chamber structures and break-downs in the blood-aqueous barrier. Changes in blood-aqueous barrier permeability could induce metabolic lens disturbances. These disturbances might accelerate cataract development, while anterior chamber inflammation could induce corneal endothelium damage and increase intraocular pressure.20 In this study, endothelial cell loss was 8.9% at six months and serious endothelial damage did not occur. Presently, new models of angle-supported phakic IOLs have been modified to avoid these problems; however, long-term studies are necessary to assess the potential risks of these new models.
The potential for intraocular pressure elevation may be related to pupillary block in the early postoperative period, with some investigators demonstrating a mean incidence of 4.8~7.2%.21,22 It is proposed that the preoperative iridectomy contributed to the prevention of intraocular pressure elevation in our study.
The pupil-related problems such as iris atrophy, iris retraction, pupil ovalization and others may be induced when large angle-supported intraocular lenses (compared to patients' corneal diameter) are inserted. Thus, an anterior chamber IOL with a flexible haptic is required to prevent these problems.23 In our study, iris complications did not occur, although the total number of cases in this study was relatively small.
Compared to phakic posterior chamber intraocular lenses, the phakic anterior chamber intraocular lens has the advantage that glare incidence is lower due to its larger optic. However, Phakic 6H® needs further improvement due to surveys of patient satisfaction level in which most patients did not give above average grades for glare and halo symptoms.
Another complication posed by the implantation of a phakic IOL is the possibility of inducing cataracts.4,22 Phakic posterior chamber intraocular lenses may contact the crystalline lens during accommodation, thus increasing the possibility of cataract formation.22,24 Lackner et al.25 reported lens opacification in 11 (14.5%) of 76 myopic eyes, and Pineda-Fernandez et al.3 reported opacification in two (11.1%) of 18 very highly-myopic eyes after implantation of phakic posterior chamber IOLs.
Amblyopia is a reduction in visual acuity as a result of a developmental disorder of spatial vision arising from strabismus, anisometropia, or from deprivation early in life.26 The following are known to cause amblyopia in very high-myopia patients: difficulty to fit accurate images on the retina through full refractive correction, small image sizes due to the lens effect, narrow visual fields, and the possibility of manifesting very high order aberrations and so forth.27
The visual system is thought to be sensitive to the effects of normal experience only during a limited period of time early in life, when it is immature and plastic. Accordingly, therapy for amblyopia does not offer significant therapeutic effects for patients over the age of 10. As such, no clear method is suggested today as a therapeutic method for adult amblyopia.28 Generally in the case of very high myopia with amblyopia, most use spectacles or contact lenses.
There have been limited efforts to improve amblyopia in adults, even slightly, because of the suspected permanency of the deficit. Nevertheless, several reports now describe the satisfactory treatment of amblyopia with improvements in BCVA in older children and adults.28-32 Wick et al.29 and Simmers and Gray30 claim that age is not a critical factor for the outbreak of amblyopia therapy, and that conventional therapy for amblyopia such as full refractive correction, occlusion therapy, prism prescription and so forth can help improve visual function in adult amblyopia patients. Sakatani et al.31 and Barequet et al.32 reported that improvement in best corrected visual acuity resulted after performing LASIK surgery on adult amblyopia patients.
In this study, we were able to improve best corrected visual acuity through phakic anterior chamber intraocular lens insertion in adult amblyopic eyes with very high myopia. The mechanism of refractive surgeries that improves vision is considered to be the effect of the magnification of the image and refinement at the corneal or lenticular plane rather than the spectacle plane.27,31 According to conventional concepts, treatment of adult amblyopia is ineffectual; but by given previous reports28-32 and this study, it is possible to assume that numerous patients are classified as amblyopia because therapy that may appropriately induce emmetropia has not been attempted in them.
This study indicates that angle-supported phakic anterior chamber IOL implantation may be an effective surgical alternative for the correction of amblyopic adult eyes with high myopia. However, long-term evaluation is needed to assess possible complications such as cataract, glaucoma, endothelial cell loss, and long-term safety.
Conflicts of interest
None of the authors have a financial or proprietary interest in any material or method mentioned.
Notes
This study was presented at the American Society of Cataract and Refractive Surgery, San Diego, CA, May 2004
REFERENCES
1. Arne JL. Phakic intraocular lens implantation versus clear lens extraction in highly myopic eyes of 30- to 50-year-old patients. J Cataract Refract Surg 2004;30:2092-2096.


2. Colin J, Robinet A, Cochener B. Retinal detachment after clear lens extraction for high myopia; seven-year follow-up. Ophthalmology 1999;106:2281-2284.


3. Pineda-Fernandez A, Jaramillo J, Vargas J, et al. Phakic posterior chamber intraocular lens for high myopia. J Cataract Refract Surg 2004;30:2277-2283.


4. Menezo JL, Peris-Martinez C, Cisneros AL, Martinez-Costa R. Phakic intraocular lenses to correct high myopia: Adatomed, Staar, and Artisan. J Cataract Refract Surg 2004;30:33-44.


5. Lyle WA, Jin GJ. Laser in situ keratomileusis with the VISX star laser for myopia over 10.0 dipoters. J Cataract Refract Surg 2001;27:1812-1822.


6. Sher NA, Barak M, Daya S. Excimer laser photorefractive keratectomy in high myopia; a multicenter study. Arch Ophthalmol 1992;110:935-943.


7. Huber C. Effectiveness of intraocular lens calculation in high ametropia. J Cataract Refract Surg 1989;15:667-672.


8. Pallikaris IG, Papatzanak ME, Stathi EZ. Laser in situ keratomileusis. Laser Surg Med 1990;10:463-468.

9. Geggel HS, Talley AR. Delayed onset keratectasia following laser in situ keratomileusis and photorefractive keratectomy. Ophthalmology 2000;107:640-652.


10. Holladay JT, Dudeja KR, Chang J. Functional vision and corneal changes after laser in situ keratomileusis determined by contrast sensitivity, glare testing, and corneal topography. J Cataract Refract Surg 1999;25:663-669.


11. Oshika T, Klyce SD, Applegate RA, et al. Comparison of corneal wavefront aberrations after photorefractive keratectomy and laser in situ keratomileusis. Am J Ophthalmol 1999;127:1-7.


12. Pesando PM, Ghiringhello MP, Tagliavacche P. Posterior chamber collamer phakic intraocular lens for myopia and hyperopia. J Refract Surg 1999;15:415-423.

13. Allemann N, Chamon W, Tanaka HM, et al. Myopic angle-supported intraocular lenses: two-year follow-up. Ophthalmology 2000;107:1549-1554.


14. The Implantable Contact Lens in Treatment of Myopia Study Group. U.S. Food and Drug Administration clinical trial of the Implantable Contact Lens for moderate to high myopia. Ophthalmology 2003;110:255-266.


15. Smith RJ. A new method of estimating the depth of the anterior chamber. Br J Ophthalmol 1979;63:215-220.



16. Waring GO 3rd, Lynn MJ, Nizam A, et al. The Perk Study Group. Results of the Prospective Evaluation of Radial Keratotomy (PERK) Study five years after surgery. Ophthalmology 1991;98:1164-1176.


17. Buratto L, Ferrari M. Indications, techniques, results, limits, and complications of laser in situ keratomileusis. Curr Opin Ophthalmol 1997;8:59-66.

18. Dutt S, Steinert RF, Raizman MB, Puliafito CA. One-year results of excimer laser photorefractive keratectomy for low to moderate myopia. Arch Ophthalmol 1994;112:1427-1436.


19. Assetto V, Benedetti S, Pesando P. Collamer intraocular contact lens to correct high myopia. J Cataract Refract Surg 1996;22:551-556.


20. Perez-Santonja JJ, Iradier MT, Benitez del Castillo JM, et al. Chronic subclinical inflammation in phakic eyes with intraocular lenses to correct myopia. J Cataract Refract Surg 1996;22:183-187.


21. Alio JL, de la Hoz F, Perez-Santonja JJ, et al. Phakic anterior chamber lenses for the correction of myopia: a 7 year cumulative analysis of complications in 263 cases. Ophthalmology 1999;106:458-466.


22. Zaldivar R, Davidorff JM, Oscherow S. Posterior chamber phakic intraocular lens for myopia of -8 to -19 diopters. J Refract Surg 1998;14:294-305.


23. Baikoff G, Arne JL, Bokobza Y, et al. Angle-fixated anterior chamber phakic intraocular lens for myopia of -7 to -19 diopters. J Refract Surg 1998;14:282-293.


24. Jean LA, Laurence CL. Phakic posterior chamber lenses for high myopia: Functional and anatomical outcomes. J Cataract Refract Surg 2000;26:369-374.


25. Lackner B, Pieh S, Schmidinger G, et al. Long-term results of implantation of phakic posterior chamber intraocular lenses. J Cataract Refract Surg 2004;30:2269-2276.


27. Applegate RA, Howland HC. Magnification and visual acuity in refractive surgery. Arch Ophthalmol 1993;111:1335-1342.


28. El Mallah MK, Chakravarthy U, Hart PM. Amblyopia: is visual loss permanent? Br J Ophthalmol 2000;84:952-956.



29. Wick B, Wingard M, Cotter S, Scheiman M. Anisometropic amblyopia: is the patient ever too old to treat? Optom Vis Sci 1992;69:866-878.


30. Simmers AJ, Gray LS. Improvement of visual function in an adult amblyope. Optom Vis Sci 1999;76:82-87.


Fig.┬Ā1
Stability of postoperative refraction. Postoperative refraction observed at 1 week remained at 1, 2, 3 and 6 months. (Repeated measures ANOVA within-subjects; p=0.83)
SE=spherical equivalent.
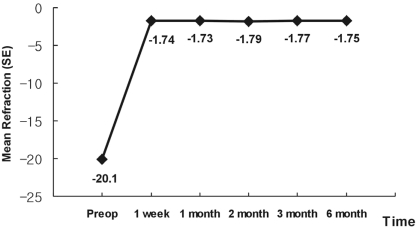
Fig.┬Ā4
Subjective six-month postoperative evaluation of quality of vision in patients with phakic anterior chamber IOLs operation.
- TOOLS




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




