 |
 |
| Korean J Ophthalmol > Volume 38(1); 2024 > Article |
|
Abstract
Purpose
In the present study, we determined the prevalence of obstructive meibomian gland dysfunction (MGD), hyposecretory MGD, grossly normal MG, and hypersecretory MGD in patients with dry eye syndrome using lipid layer thickness (LLT) and MG dropout.
Methods
Eighty-eight patients with dry eye syndrome were included in the study. Patients were categorized into four groups according to the LLT and weighted total meiboscore. The proportion of patients in each group was calculated. The age, sex, Ocular Surface Disease Index, LLT, Schirmer, tear film breakup time, cornea stain, weighted total meiboscore, expressibility, and quality of meibum were compared between the four groups.
Results
Fifteen eyes (17.0%) had obstructive MGD, two eyes (2.3%) had hyposecretory MGD, 40 eyes (45.5%) had grossly normal MG, and 17 eyes (19.3%) had hypersecretory MGD. The obstructive MGD group was younger than the grossly normal MG group. In obstructive MGD, the ratio of men to women was higher than that of the other groups. However, Ocular Surface Disease Index, Schirmer, tear film breakup time, and corneal stain did not show statistically significant differences between the four groups. The meibum expressibility of the hyposecretoy MGD group was worse than those of the other groups. The meibum expressibility of the hyposecretoy MGD group was poor than those of the obstructive and hypersecretory MGD group.
Lipid layer thickness (LLT) measurement using tear film interferometry such as LipiView (Johnson & Johnson Vision) has been recently used for the diagnosis of dry eye syndrome [1-6]. There are two major complaints from clinicians about LLT. First, the reproducibility is too low; second, clinicians feel that LLT is not related to other clinical parameters. For example, even when the meibomian gland (MG) loss (dropout) shown in meibography is very severe, the lipid layer is sometimes very thick. Even when meibography shows minimal MG loss, the lipid layer is sometimes very thin. In 2013, Eom et al. [7] reported that upper or lower MG losses showed a significant negative correlation with LLT in the obstructive MG dysfunction (MGD) group as well as in the control group, but there is a large discrepancy between the report and real clinical experience. We believe that we can determine the status of the MG by combination of LLT and MG loss rather than considering the absence of any significant relationship between LLT and MG loss in the patients. Meibography shows the morphologic changes of MGs. Tear film interferometry measures the thickness of the tear film lipid layer formed by meibum secreted from the meibomian gland, so LLT shows the function of MGs. If LLT is too thin and MG loss is minimal, we can determine that there is an obstructive MGD. If LLT is too thin and MG loss is severe, we can determine the presence of a hyposecretory MGD. If LLT is normal and MG loss is minimal, we can determine that there is grossly normal MG. If LLT is too thick regardless of MG loss, we can determine the presence of a hypersecretory MGD. The purpose of this study was to determine the prevalence of obstructive MGD, hyposecretory MGD, absence of MGD, and hypersecretory MGD in patients with dry eye syndrome, according to these criteria.
This study followed the principles of the Declaration of Helsinki. The study was approved by the Institutional Review Board of Yeouido St. MaryŌĆÖs Hospital (No. SC20RISI0064). The requirement for informed consent was waived due to the retrospective nature of the study.
This study is a retrospective chart review of 88 patients who visited Blue Eye Center (Seoul, Korea) for dry eye syndrome. The inclusion criteria for the dry eye group were symptoms typical for this syndrome (i.e., dryness, itching, foreign body sensation) with a low tear film breakup time (tBUT; 5 seconds), low Schirmer I score (<10 mm/5 min without anesthesia), and corneal punctate fluorescein staining (Oxford staining score [8] of >1) in either eye. Exclusion criteria included a history of ocular injury, infection, non-dry eye ocular inflammation, trauma, surgery within the previous 6 months, uncontrolled systemic disease, or contamination of the tear film lipid layer by cosmetics (oil, lotion, and sun cream). In addition, all the video recorded by LipiView was reviewed by an MGD expert (HSH). If the video was so blurry that we could not judge the contamination by cosmetics, we excluded the patient from the study.
The tests were performed as follows. First, subjective symptoms were graded on a numerical scale from 0 to 4, according to the validated 12-item Ocular Surface Disease Index (OSDI) questionnaire [9]. LLT was measured with a LipiView. LipiView cannot measure LLT above 100 nm and record it just as ŌĆ£above 100 nm.ŌĆØ In this study, we determined ŌĆ£above 100 nmŌĆØ as 100 nm. Schirmer test, tBUT measurement, and a cornea stain were performed. Finally, noncontact infrared meibography was performed on the MGs in the upper and lower eyelids, using LipiView (noncontact infrared meibography). We applied fluorescein dye to both eyes to measure tBUT and scored the corneal staining according to the Oxford scoring scheme [8], using fluorescein papers (Haag-Streit). We applied Schirmer I test to both eyes without topical anesthesia. We waited for 15 minutes between these two measurements to minimize the influence of Schirmer test on the measurement of tBUT.
MG dropout in meibography was graded 0 to 3 according to meiboscore [10]. Meibomian gland dropout was graded by an MGD expert (HSH). We defined ŌĆ£weighted total meiboscoreŌĆØ as ŌĆ£2 ├Ś upper MG meiboscore + lower MG meiboscore,ŌĆØ using the fact that meibomian glands in the upper eyelid comprise about twice the volume of that in the lower eyelid [11]. The minimum of a weighted total meiboscore is 0, and the maximum of a weighted total meiboscore is 9.
In addition, the expressibility and quality of meibum during digital expression has been graded. The expressibility was graded as 0 (all glands expressible), 1 (3-4 glands expressible), 2 (1-2 glands expressible), and 3 (no glands expressible) [12]. The quality was graded as 0 (clear fluid), 1 (cloudy fluid), 2 (cloudy particulate fluid), and 3 (inspissated, like toothpaste) [13].
Patients were categorized into four groups, according to the lipid layer thickness and weighted total meiboscore: obstructive MGD, ŌĆ£LLT < mean - standard deviationŌĆØ and ŌĆ£weighted total meiboscore of 3 or lessŌĆØ; hyposecretory MGD, ŌĆ£LLT < mean - standard deviationŌĆØ and ŌĆ£weighted total meiboscore of 6 or moreŌĆØ; grossly normal MG, ŌĆ£mean - standard deviation < LLT < mean + standard deviationŌĆØ and ŌĆ£weighted total meiboscore of 3 or lessŌĆØ; hypersecretory MGD, ŌĆ£LLT > mean + standard deviation.ŌĆØ
Data from only the right eyes of 88 patients were analyzed. The sex ratio of the patients was obtained. The mean and standard deviation of age, OSDI, LLT, Schirmer, tBUT, corneal stain, and weighted total meiboscore were calculated. The histogram of the LLT was obtained, and the normality test was performed by the Shapiro-Wilk test. Multiple linear regression analysis was performed to determine the effect of age and sex on LLT. The Mann-Whitney U-test was performed to determine the difference in LLT between men and women. Correlation analysis using the Spearman test between LLT and the weighted total meiboscore was performed. The patients were categorized into four groups (obstructive MGD, hyposecretory MGD, grossly normal MG, and hypersecretory MGD), according to the abovementioned criteria. The number and proportion of patients in each group was calculated. Analysis of variance (ANOVA) was performed to compare age, OSDI, LLT, Schirmer, tBUT, cornea stain, weighted total meiboscore, meibum expressibility and quality of meibum between the four groups. The sex ratio of each group was compared by the Pearson chi-squared test. We used IBM SPSS ver. 24.0 (IBM Corp) for the statistical analysis. Results were considered statistically significant if the p-value was <0.05.
Table 1 shows the demographics and clinical parameters of 88 right eyes of 88 patients included in this study. The mean LLT was 64.6 nm, and the standard deviation was 23.9 nm. The mean weighted total meiboscore was 1.8 and the standard deviation was 2.2.
Fig. 1 is a histogram showing the distribution of LLT in 88 patients. This histogram showed an abnormally high frequency in the 100 nm region because we determined ŌĆ£above 100 nmŌĆØ as 100 nm. The normality was not satisfied by the Shapiro-Wilk test (p < 0.001).
Multiple linear regressions were used to analyze the effect of age and sex on LLT. Age did not affect LLT, but sex had a significant effect (p = 0.018) on LLT (Table 2). The LLTs were 55.5 ┬▒ 21.7 nm in 29 men and 69.0 ┬▒ 23.8 nm in 59 women, and the LLT was significantly thicker in women than in men (Mann-Whitney U-test, p = 0.014).
Fig. 2 shows the correlation between the LLT and weighted total meiboscore. In the correlation analysis, there was no significant correlation between them (Spearman rho = ŌłÆ0.097, p = 0.370).
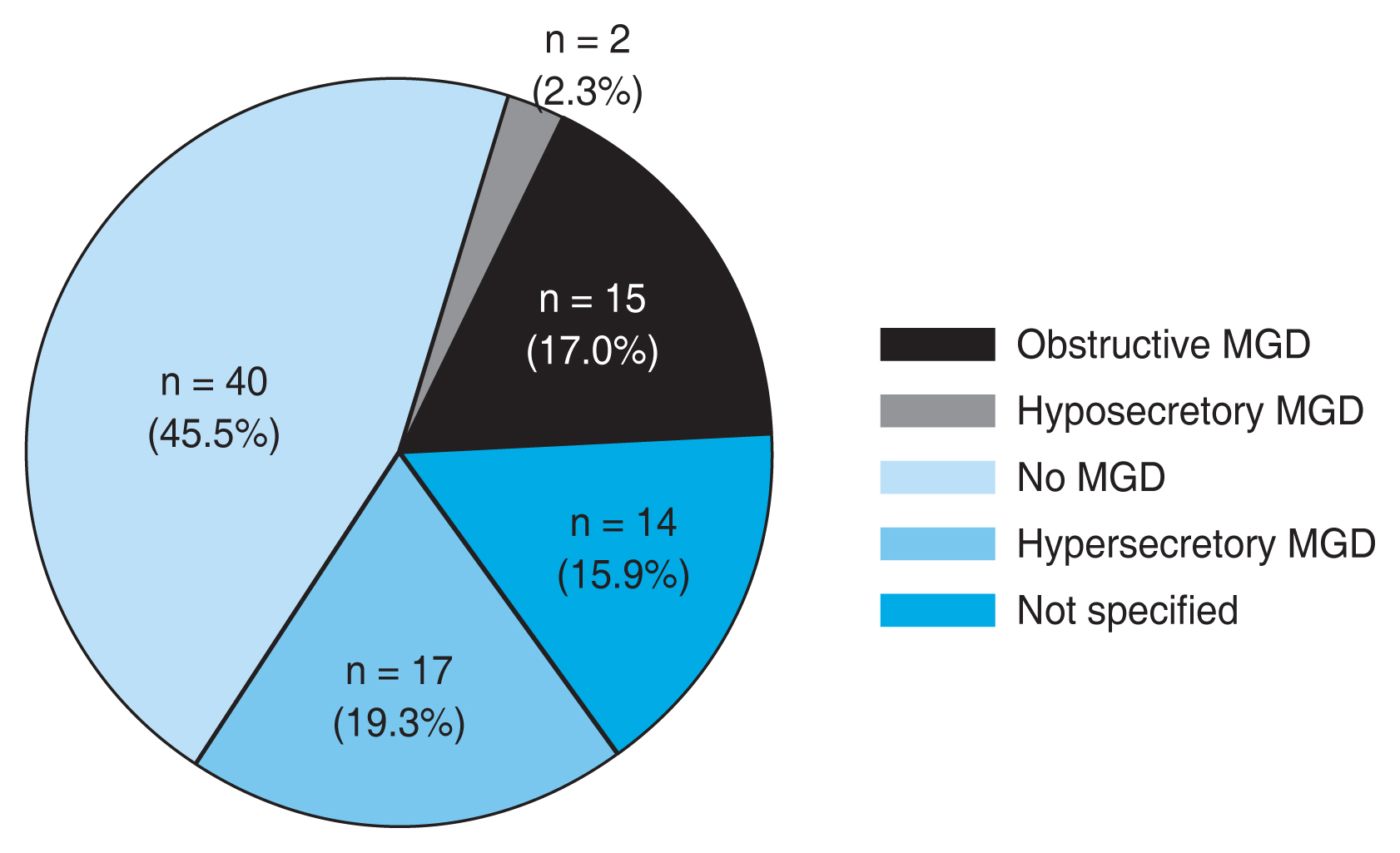
Eighty-eight eyes were categorized into four groups, according to the abovementioned criteria (Fig. 3). Fig. 4 shows the number of patients in each group. Fifteen eyes (17.0%) had obstructive MGD, two eyes (2.3%) had hyposecretory MGD, 40 eyes (45.5%) had grossly normal MG, and 17 eyes (19.3%) had hypersecretory MGD. Fourteen eyes (15.9%) were not specified.
We compared the age, sex, OSDI, LLT, Schirmer, tBUT, corneal stain, weighted total meiboscore, and meibum expressibility and quality between the four groups (Table 3). The mean age of the four groups was significantly different (ANOVA, p = 0.019). In the post hoc test, the obstructive MGD group was younger than the grossly normal MG group (Tukey test, p = 0.034). There was a significant difference in gender (Pearson chi-square test, p = 0.038). In obstructive MGD, the ratio of men to women was higher than that of the other groups. In hypersecretory MGD, the ratio of women to men was higher than that of the other groups. However, OSDI, Schirmer, tBUT, and corneal stain did not show statistically significant differences between the four groups.
The LLT of the four groups was significantly different (ANOVA, p < 0.001). In the post hoc test, there is no significant difference between obstructive MGD and hyposecretory MGD (Tukey test, p = 0.675), but there were significant differences between the other groups (obstructive MGD vs. grossly normal MG, p < 0.001; obstructive MGD vs. hypersecretory MGD, p < 0.001; hyposecretory MGD vs. grossly normal MG, p < 0.001; hyposecretory MGD vs. hypersecretory MGD, p < 0.001; grossly normal MG vs. hypersecretory MGD, p < 0.001).
The mean meibum expressibility of the four groups was significantly different (ANOVA, p = 0.033). In the post hoc test, the meibum expressibility of the hyposecretoy MGD group was worse than those of the obstructive MGD group (Tukey test, p = 0.023), grossly normal MG group (Tukey test, p = 0.027), and hypersecretory MGD group (Tukey test, p = 0.021). The mean meibum quality of the four groups was significantly different (ANOVA, p = 0.022). In the post hoc test, the meibum expressibility of the hyposecretoy MGD group was poor than those of the obstructive MGD group (Tukey test, p = 0.031) and hypersecretory MGD group (Tukey test, p = 0.022).
In this study, we categorized the patients with dry eye syndrome into obstructive MGD, hyposecretory MGD, grossly normal MG, and hypersecretory MGD, using the meibomian gland dropout in meibography and LLT measured by tear film interferometry. This is the first study to discover the prevalence of obstructive MGD, hyposecretory MGD, grossly normal MG, and hypersecretory MGD in dry eye patients.
In this study, 45.5% of patients with dry eye syndrome did not have MGD. Unexpectedly, the prevalence of hyposecretory MGD with severe meibomian gland loss and thin lipid layer was very low. The prevalence of hypersecretory MGD with a thick lipid layer was as high as 19.3%, and the prevalence of obstructive MGD was 17.0%. In 2012, Lemp et al. [14] reported the distribution of patients with aqueous deficient or an evaporative subtype of dry eye syndrome in dry eye patients. Schirmer tests and MGD grading scores were used as markers in the present study to represent aqueous-deficient dry eye and evaporative dry eye subsets of dry eye, respectively. Of the 224 dry eye patients, 79 were classified as having pure evaporative dry eye, whereas only 23 could be classified as purely aqueous deficient, and 57 patients showed evidence of both MGD and aqueous deficiency (mixed subtype). Sixty-five subjects showed no signs of aqueous tear deficiency or MGD. Therefore, 88 out of 224 patients (39.3%) showed grossly normal MG. This is similar to our results. In this study, 45.5% of patients with dry eye syndrome did not have MGD.
In 2011, the International Workshop on Meibomian Gland Dysfunction: Report of the Definition and Classification Subcommittee [15] described obstructive MGD as probably the most common form of MGD. However, in this study, the prevalence of obstructive MGD was 17.0%, and the prevalence of hypersecretory MGD was as high as 19.3%. This is more consistent with our clinical experiences. Fourteen eyes (15.9%) were not specified. In Fig. 3, these 15.9% are the patients with a high weighted total meiboscore of 6 or higher and a normal LLT, and the patients with a weighted total meiboscore of 4 or 5 and a normal or thin LLT. In the patients with a weighted total meiboscore of over 6 and normal LLT, the remaining MGs appear to be performing a hypersecretory function to compensate for the lost meibomian glands. This is a situation we often encounter in clinics, where there is significant dropout, but when the eyelids are squeezed, a significant amount of meibum is secreted. In the patients with a weighted total meiboscore is 4 or 5, which is a case where dropout is neither completely absent nor very severe, if the remaining meibomian glands area hypersecretory state, LLT can be normal. If the remaining meibomian glands are normal or hyposecretory state, LLT can be thin.
Of course, in this study, the criteria for obstructive MGD, hyposecretory MGD, grossly normal MG, and hypersecretory MGD were arbitrary, and proportions will vary according to the criteria. Even so, it may be true that there are not so many hyposecretory MGD among dry eye patients and about 50% of dry eye patients do not have MGD according to the distribution in Fig. 2.
Theoretically, MG loss is thought to reduce the LLT, but clinicians often encounter cases of discrepancies. Therefore, many doctors suspect the reliability of tear film interferometry or do not think it is clinically helpful. In the present study, there was no significant correlation between LLT and loss of meibomian gland. The reason for this is that patients with dry eye syndrome that we examined had heterogeneous status of meibomian glands: obstructive MGD, hyposecretory MGD, grossly normal MG, or hypersecretory MGD.
The treatment of MGD should depend on the subtype of MGD. In the case of obstructive MGD, a warm compress such as LipiFlow is an important treatment. In the case of hyposecretory MGD, a lipid substitute eye drop is an important treatment instead of warm compress. In cases of aqueous-deficient dry eye without MGD, artificial tears and punctal plug are the main treatments. In the cases of hypersecretory MGD, lid hygiene and systemic antibiotics such as doxycycline and tetracycline will be the main treatment. Therefore, the LLT measurement and meibography should be performed to determine the MG status and the treatment modality.
LipiView is a device capable of LLT measurement and meibography. However, for the reliability of the test, the LLT measurement should be performed before meibography. During meibography, manipulation of the eyelids (eyelid eversion) causes excessive meibum expression. If the LLT is measured after meibography, it will be overestimated. In this study, we measured the LLT before meibography in all cases.
There was no significant difference in the clinical parameters (OSDI, Schirmer, tBUT, cornea stain) except age and sex between the four groups. The age of the patients with obstructive MGD was younger than that in the grossly normal MG group, perhaps because obstructive MGD is defined as a weighted total meiboscore of 3 or less, and the younger the patients are, the lower the meiboscores are [10]. In obstructive MGD, the ratio of men to women was higher than that of the other groups. In hypersecretory MGD, the ratio of women to men was higher than that of the other groups. This may be related to the thicker lipid layer in women than in men. There was no significant difference between the four groups in terms of Schirmer, tBUT, and cornea stain, which suggests that these clinical parameters are explainable not only with a MGD category.
In this study, the lipid layer was thicker in women than in men. This is similar to a report that measured LLT by using LipiView in dry eye patients [4]. Sometimes, the cosmetics contamination in women causes overestimation of the LLT. However, in this study, all the videos were reviewed to eliminate this possibility by excluding cases when cosmetic contamination was suspected or videos were blurred and difficult to judge them. Still, LLT was thicker in women, and we do not know the exact reason for that.
Categorization of MG status using only LLT and meibography may be insufficient. It is also necessary to check the lid margin abnormality and the quality of the meibum secreted by lid squeezing. However, the lipid layer on the tear film reflects a more physiologic state than the meibum secreted by lid squeezing. In this study, we have categorized the MG status with only the LLT indicating the function of the MG and the meiboscore indicating the morphology of the MG.
The weakness of this research is that we performed only the Schirmer test and did not perform tear meniscus optical coherence tomography for tear volume. If we had performed it, it would be easy to judge the aqueous deficiency exactly. Additionally, because the number of patients (88) is relatively small, the prevalence of each MGD category may be not accurate. If the number of subjects is increased in future research, a more accurate prevalence estimate will be possible.
We could categorize the dry eye patients by the condition of obstructive MGD, hyposecretory MGD, grossly normal MG, and hypersecretory MGD, using LLT and meibography and determined the proportion of them in dry eye syndrome patients. This categorization may be helpful to determine the best treatment method for dry eye syndrome, according to the MG status.
Notes
Funding
This study was supported by the Korea Health Technology R&D Project grant through the Korea Health Industry Development Institute (KHIDI), funded by the Korean Ministry of Health and Welfare (No. HI17C0659); and by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Korean Ministry of Education (No. 2017R1A1A2A 10000681, 2020R1A2C1005009). The funder had no influence on the design, collection, analysis, or interpretation of the data, or in writing the manuscript.
References
1. Zhao Y, Tan CL, Tong L. Intra-observer and inter-observer repeatability of ocular surface interferometer in measuring lipid layer thickness. BMC Ophthalmol 2015;15:53.




2. Satjawatcharaphong P, Ge S, Lin MC. Clinical outcomes associated with thermal pulsation system treatment. Optom Vis Sci 2015;92:e334-41.


3. Kim JS, Lee H, Choi S, et al. Assessment of the tear film lipid layer thickness after cataract surgery. Semin Ophthalmol 2018;33:231-6.


4. Jung JW, Park SY, Kim JS, et al. Analysis of factors associated with the tear film lipid layer thickness in normal eyes and patients with dry eye syndrome. Invest Ophthalmol Vis Sci 2016;57:4076-83.


5. Finis D, Pischel N, Schrader S, Geerling G. Evaluation of lipid layer thickness measurement of the tear film as a diagnostic tool for Meibomian gland dysfunction. Cornea 2013;32:1549-53.


6. Hwang H, Jeon HJ, Yow KC, et al. Image-based quantitative analysis of tear film lipid layer thickness for meibomian gland evaluation. Biomed Eng Online 2017;16:135.




7. Eom Y, Lee JS, Kang SY, et al. Correlation between quantitative measurements of tear film lipid layer thickness and meibomian gland loss in patients with obstructive meibomian gland dysfunction and normal controls. Am J Ophthalmol 2013;155:1104-10.


8. Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003;22:640-50.


9. Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol 2000;118:615-21.


10. Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology 2008;115:911-5.


11. Greiner JV, Glonek T, Korb DR, et al. Volume of the human and rabbit meibomian gland system. Adv Exp Med Biol 1998;438:339-43.


12. Pflugfelder SC, Tseng SC, Sanabria O, et al. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea 1998;17:38-56.


13. Bron AJ, Benjamin L, Snibson GR. Meibomian gland disease: classification and grading of lid changes. Eye (Lond) 1991;5(Pt 4):395-411.



Fig.┬Ā1
A histogram showing the distribution of lipid layer thickness in 88 patients. This histogram showed an abnormally high frequency in the 100 nm region because we determined ŌĆ£above 100 nmŌĆØ as 100 nm. The normality was not satisfied by the Shapiro-Wilk test (p < 0.001).
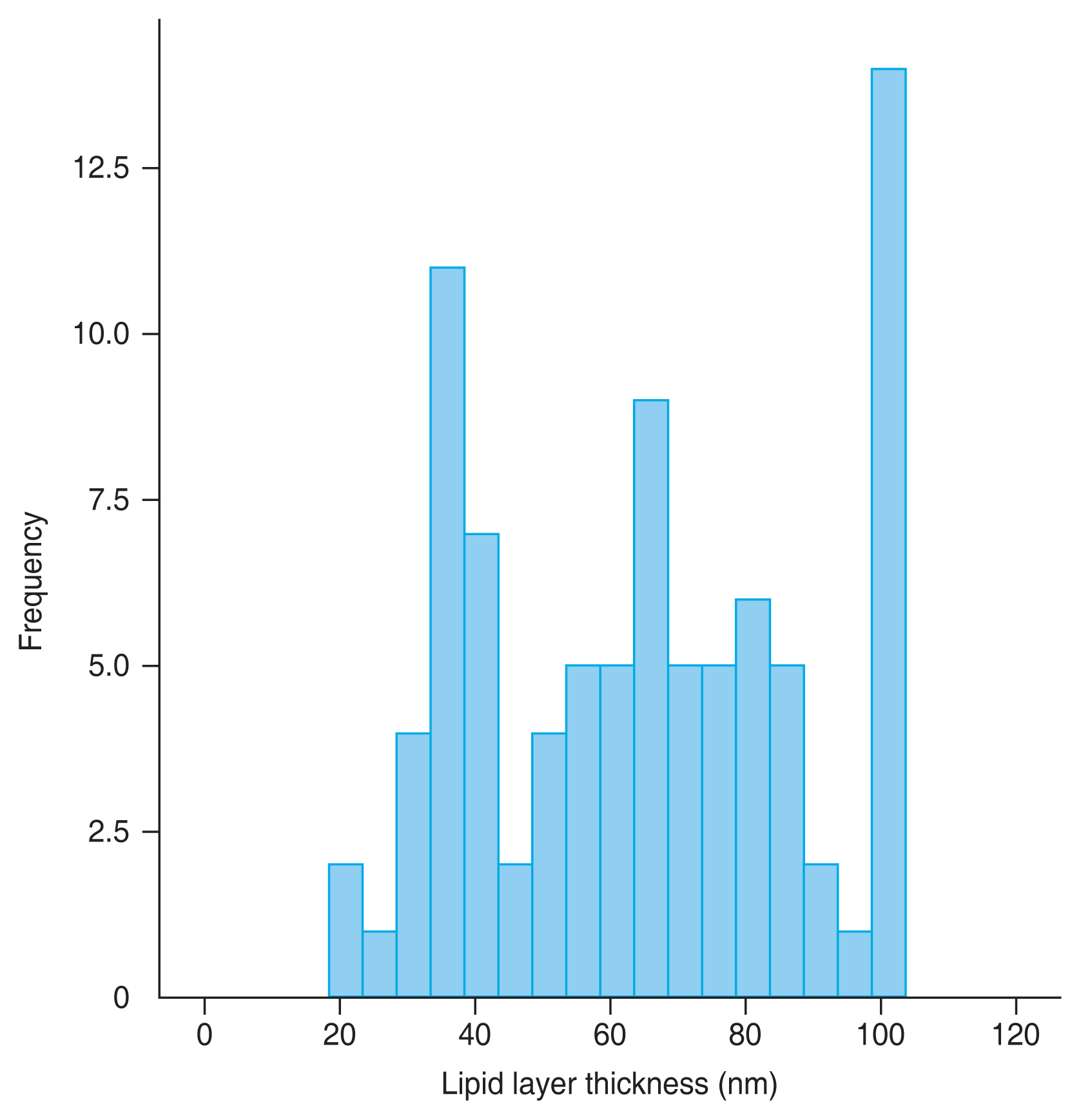
Fig.┬Ā2
Correlation between the lipid layer thickness and weighted total meiboscore. In the correlation analysis, there was no significant correlation between them (Spearman rho = ŌłÆ0.097, p = 0.370).
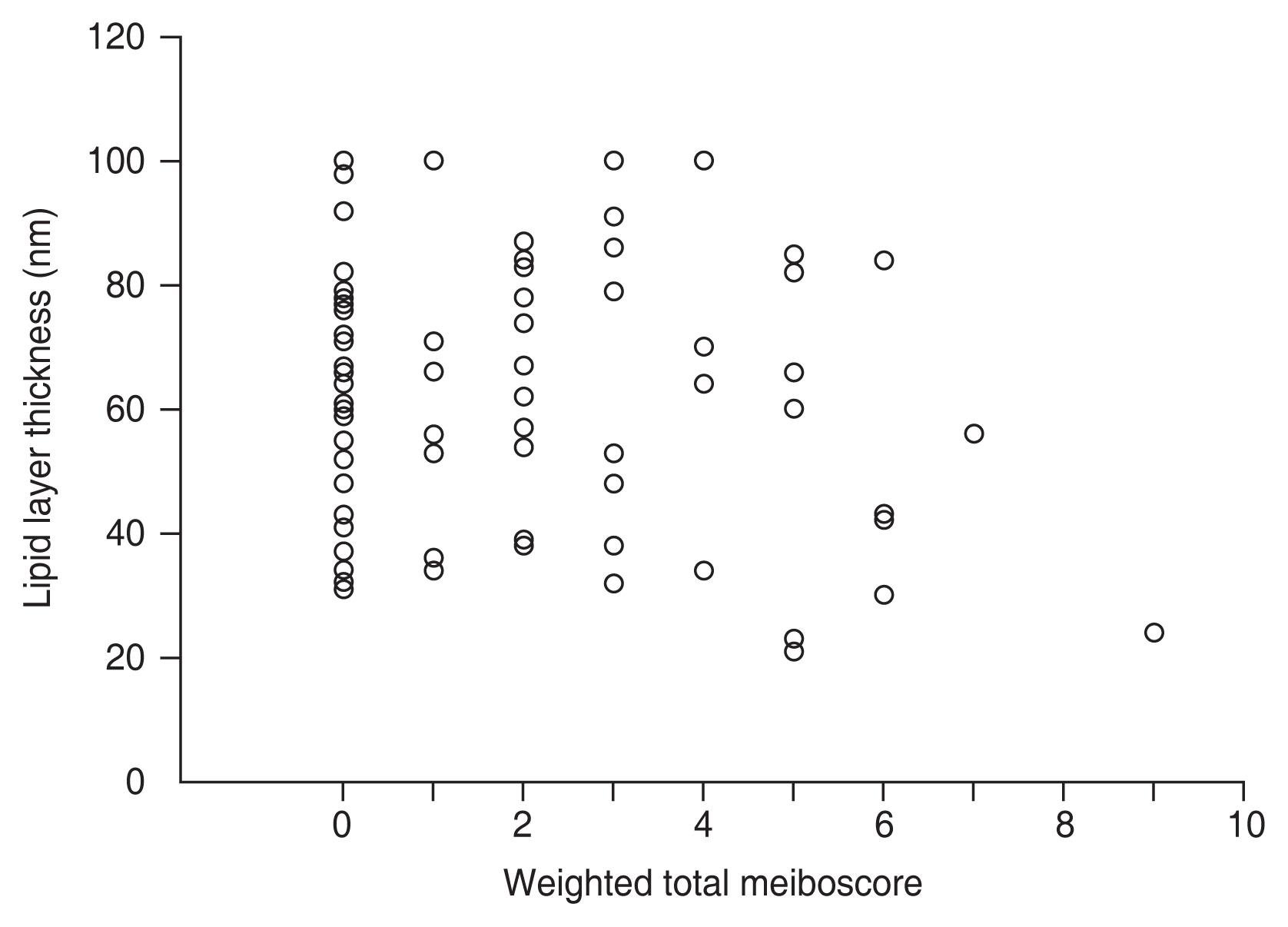
Fig.┬Ā3
Categorization of meibomian gland dysfunction (MGD) using lipid layer thickness (LLT) and MG dropout. Eighty-eight eyes were categorized into four groups, according to the criteria: (1) obstructive MGD, ŌĆ£(LLT < mean - standard deviation [SD]) and (weighted total meiboscore of 3 or less)ŌĆØ; (2) hyposecretory MGD, ŌĆ£(LLT < mean - SD) and (weighted total meiboscore of 6 or more)ŌĆØ; (3) grossly normal MG, ŌĆ£(mean - SD < LLT < mean + SD) and (weighted total meiboscore of 3 or less)ŌĆØ; and (4) hypersecretory MGD, ŌĆ£(LLT > mean + SD).ŌĆØ
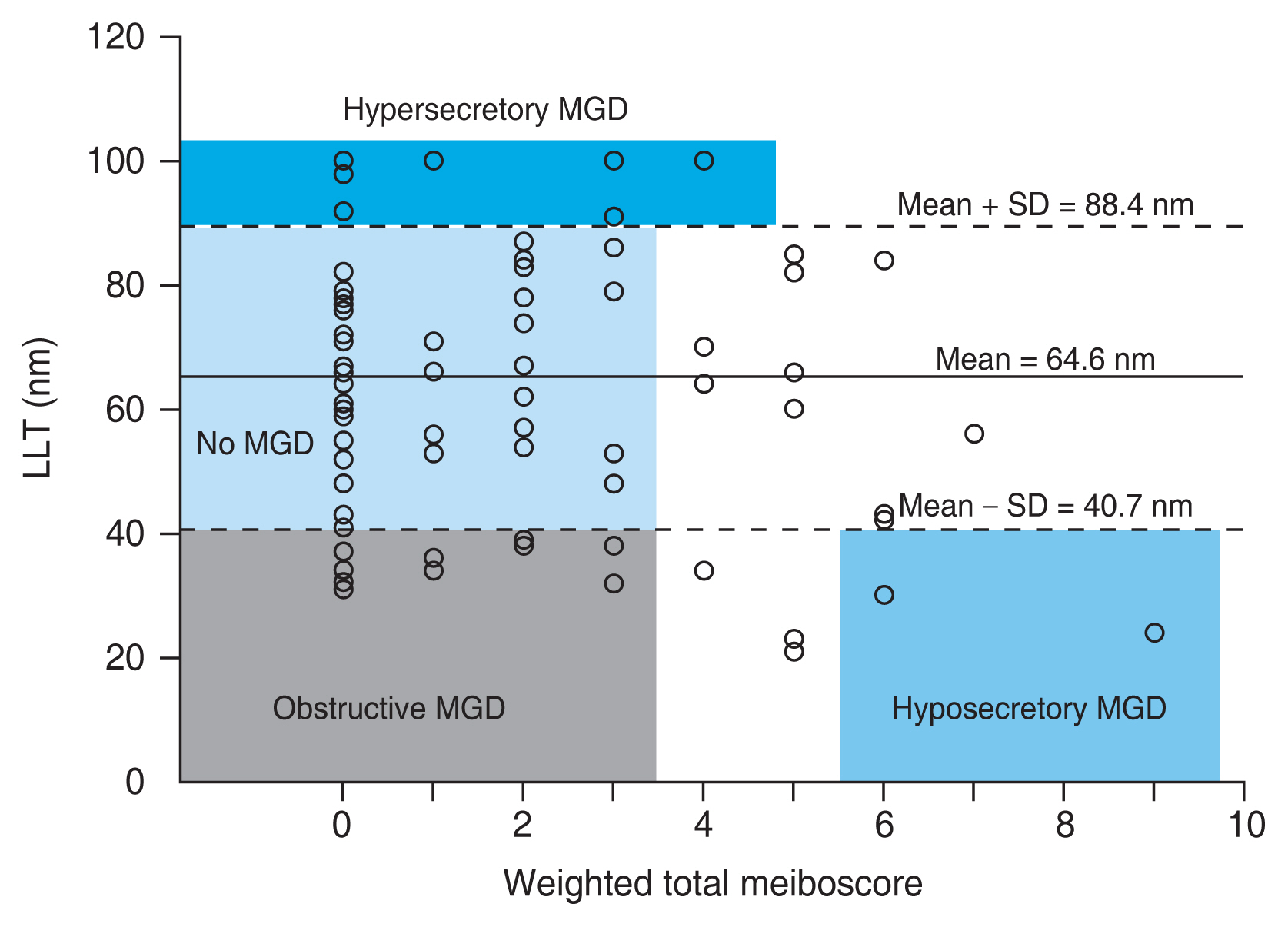
Fig.┬Ā4
A pie chart showing the number of patients in each group. Fifteen eyes (17.0%) had obstructive meibomian gland dysfunction (MGD), two eyes (2.3%) had hyposecretory MGD, 40 eyes (45.5%) had grossly normal MG, and 17 eyes (19.3%) had hypersecretory MGD. Fourteen eyes (15.9%) were not specified.
Table┬Ā1
Demographics and clinical parameters of 88 right eyes from 88 patients included in this study
Table┬Ā2
Multiple linear regression analyzing the effect of age and sex on lipid layer thickness
| Variable | Unstandardized coefficient | Standardized coefficient | t | p-value | |
|---|---|---|---|---|---|
|
|
|||||
| ╬▓ | Standard error | ||||
| Constant | 34.490 | 10.786 | - | 3.198 | 0.002 |
| Age | 0.198 | 0.153 | 0.135 | 1.295 | 0.199 |
| Sex | 12.653 | 5.264 | 0.251 | 2.404 | 0.018 |
Table┬Ā3
Comparison of clinical parameters between four groups
| Parameter | Obstructive MGD (n = 15) | Hyposecretory MGD (n = 2) | No MGD (n = 40) | Hypersecretory MGD (n = 17) | p-value |
|---|---|---|---|---|---|
| Age (yr) | 32.9 ┬▒ 13.1 | 58.5 ┬▒ 0.7 | 45.4 ┬▒ 16.7 | 45.2 ┬▒ 11.7 | 0.019* |
| Sex | 0.038ŌĆĀ | ||||
| ŌĆāMale | 9 | 0 | 11 | 3 | |
| ŌĆāFemale | 6 | 2 | 29 | 14 | |
| Ocular Surface Disease Index | 49.6 ┬▒ 28.9 | 32.9 ┬▒ 33.1 | 48.8 ┬▒ 25.7 | 48.8 ┬▒ 25.8 | 0.866* |
| Lipid layer thickness (nm) | 35.1 ┬▒ 2.6 | 27.0 ┬▒ 4.2 | 66.2 ┬▒ 12.5 | 98.9 ┬▒ 2.8 | <0.001 |
| Schirmer (mm) | 11.2 ┬▒ 7.0 | 11.5 ┬▒ 3.5 | 9.6 ┬▒ 6.6 | 9.3 ┬▒ 6.8 | 0.824* |
| Tear breakup time (sec) | 3.0 ┬▒ 0.9 | 5.0 ┬▒ 1.1 | 3.8 ┬▒ 1.7 | 3.6 ┬▒ 1.4 | 0.172* |
| Corneal stain (Oxford) | 0.8 ┬▒ 0.7 | 1.0 ┬▒ 1.4 | 1.0 ┬▒ 0.8 | 1.3 ┬▒ 1.0 | 0.426* |
| Weighted total meiboscore | 0.9 ┬▒ 1.2 | 7.5 ┬▒ 2.1 | 0.9 ┬▒ 1.1 | 1.3 ┬▒ 1.6 | <0.001 |
| Meibum expressibility | 1.1 ┬▒ 0.6 | 2.5 ┬▒ 0.7 | 1.2 ┬▒ 0.7 | 1.1 ┬▒ 0.7 | 0.033 |
| Meibum quality | 1.0 ┬▒ 0.8 | 2.5 ┬▒ 0.7 | 1.3 ┬▒ 0.7 | 0.9 ┬▒ 0.7 | 0.022 |



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print




