Subfoveal Nodule Affecting Visual Prognosis in Coats Disease
Article information
Abstract
Purpose
To investigate the characteristics of subfoveal nodules in Korean patients with Coats disease and their association with visual outcomes
Methods
A retrospective analysis was conducted within the medical records of patients with stage 2B or 3A1 Coats disease, including clinical features, imaging, presence of either a subfoveal nodule or macular fibrosis, and visual outcome.
Results
Twelve patients were present with stage 2B or 3A1 Coats disease, and nine patients (75%) presented with subfoveal nodule. Between the group without subfoveal nodule and the group with subfoveal nodule, there were no significant differences in age (mean, 14.0 ± 1.7 years vs. 27.7 ± 21.8 years; p = 0.482), sex (all men), stage of the disease (stage 2B: three patients vs. eight patients, p > 0.999; stage 3A1: none vs. one patient, p > 0.999), extension of retinal exudation (mean, 7.7 hours vs. 4.1 hours; p = 0.209) and peripheral telangiectasia (mean, 3.7 hours vs. 4.2 hours; p = 0.727), and follow-up duration (mean, 65.0 months vs. 46.1 months; p = 0.600). There were significantly more patients with severe visual loss (≤20 / 200) among the patients with subfoveal nodule (none vs. seven patients, p = 0.045), and the cause for severe visual loss was macular fibrosis in all cases. Macular fibrosis developed significantly more frequently in the patients with subfoveal nodule (none vs. seven = patients, p = 0.045).
Conclusions
This study is the first study covering the analysis of subfoveal nodules in Korean patients with Coats disease. The existence of a subfoveal nodule at the initial diagnosis serves as an indicator predicting the development of macular fibrosis and a less favorable visual outcome in the patients with Coats disease. A multicenter study with a larger patient pool and further studies toward the therapeutic approach for the subfoveal nodule and macular fibrosis are needed.
Coats disease was first described by George Coats in 1908 [1]. This rare, nonhereditary disorder predominantly affects young male and typically manifests unilaterally. Coats disease is characterized by the presence of abnormal, leaky blood vessels that can lead to the accumulation of fluid and lipids in the retina. This accumulation can cause decreased visual acuity and, in progressed cases, even blindness. Early detection and intervention are crucial in managing Coats disease to preserve vision and prevent complications. Treatment options typically include laser therapy or cryotherapy to seal off the abnormal blood vessels and reduce leakage, as well as other surgical procedures depending on the severity of the condition.
According to Shields et al. [2], when foveal exudates are absent at the initial assessment, a more favorable visual prognosis is expected, while the presence of thick foveal exudation typically indicates a worse functional outcome. Moreover, Shields et al. [2] have noted that poor vision is likely to be identified when the exudation presents as a fibroglial nodule in the subfoveal area, which can be referred to the concept including subfoveal nodule [3] and macular fibrosis [4], while this notion was not reflected in their classification system.
This study’s objective is to investigate the characteristics of subfoveal nodules in Korean patients with Coats disease, as well as their association with long-term visual outcomes.
Materials and Methods
Ethics statement
This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (No. B-2310-861-105) with a waiver of informed consent due to its minimal risk to subjects. The study was performed in accordance with the tenets of the Declaration of Helsinki.
Study design
We retrospectively reviewed the medical records of patients diagnosed with Coats disease between March 2003 and August 2023 at Seoul National University Bundang Hospital (Seongnam, Korea). General data included patient age, sex, and laterality of Coats disease. Visual acuities, tonometry measures, and findings from slit-lamp examination at presentation and for each subsequent visit were noted. Fundus examination data were obtained from color fundus photographs with Optos California (Optos PLC). The amount of peripheral retinal exudation and telangiectasia at presentation was quantified in terms of clock hours, from 1 to 12.
Shields et al. [2] have introduced a classification system based on a retrospective review of 150 cases of Coats disease, to predict visual prognosis based on the presence of foveal exudation and exudative retinal detachment at the initial diagnosis: stage 1, telangiectasia only; stage 2, telangiectasia and exudation (2A, extrafoveal exudation; 2B, foveal exudation); stage 3, exudative retinal detachment (3A1, extrafoveal subtotal retinal detachment; 3A2, foveal subtotal retinal detachment; 3b, total retinal detachment); stage 4, total detachment and glaucoma; and stage 5, advanced end-stage disease. The patients’ stages of the disease were classified according to the classification system by Shields et al. [2] into stages 1, 2A, 2B, 3A1, 3A2, 3B, 4, and 5.
The review also included the identification of either a subfoveal nodule or macular fibrosis. A subfoveal nodule was characterized as a spheroidal lesion with a yellow, exudative, and protruding appearance. (Fig. 1A–1C) Macular fibrosis was characterized as a subretinal white-gray scar at the fovea identified in Optos and optical coherence tomography [5]. Additionally, findings from fluorescein angiography (FA) performed using Optos were recorded, and the presence of retinal–retinal anastomosis or neovascularization of the retina and choroid was determined. Data from spectral-domain optical coherence tomography (SDOCT) obtained with Spectralis (Heidelberg Engineering) were also documented. Patients without foveal exudation or with foveal exudative retinal detachment at presentation were excluded from the study.
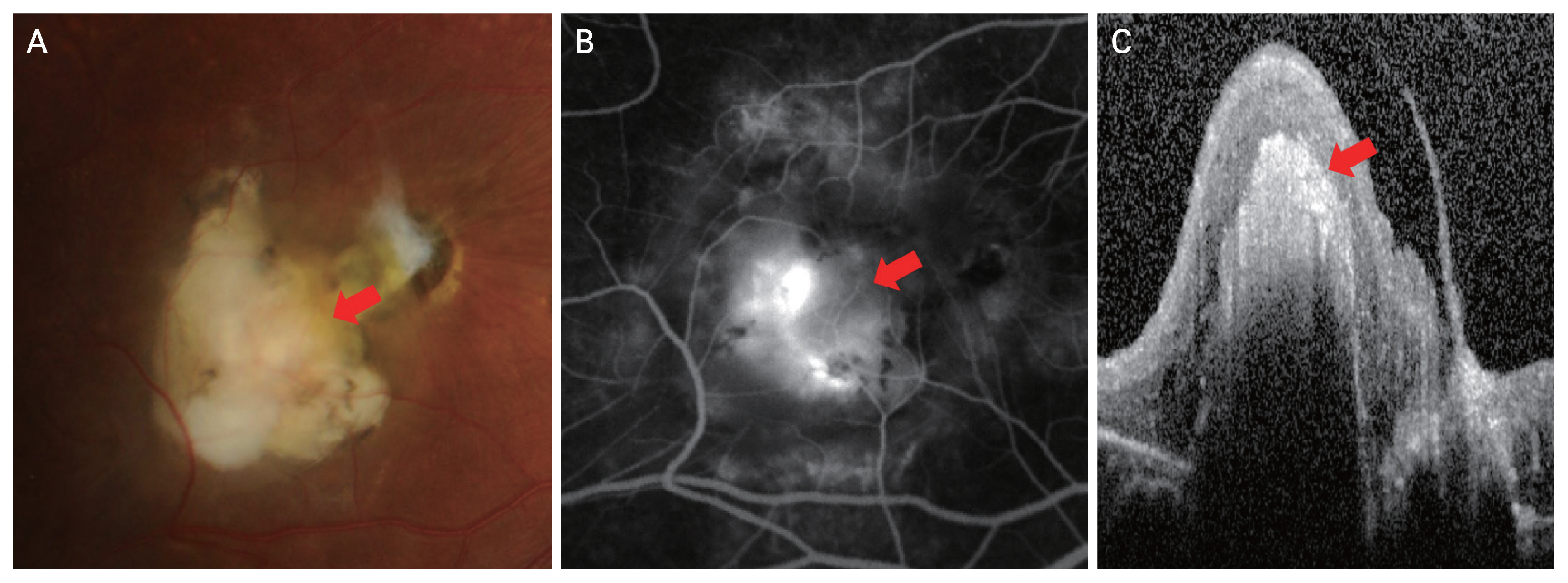
A 9-year-old male patient presenting with stage 2B Coats disease. (A) Color fundus photograph showed a subfoveal nodule (arrow). (B) Fluorescein angiography showed a hyperfluorescent subfoveal lesion during the late phase (arrow). (C) Spectral-domain optical coherence tomography showed a highly reflective subfoveal lesion, accompanied by a shadowing effect (arrow).
Statistical analysis
Comparative analyses were performed between eyes with or without subfoveal nodule. Baseline clinical characteristics were compared using IBM SPSS ver. 19.0 (IBM Corp). Visual acuity levels were reported in decimal unit and the logarithm of the minimal angle of resolution was used to calculate means. Statistical significance was set at p < 0.05. The statistical significances for the mean age, mean retinal exudation, mean peripheral telangiectasia, and mean follow-up duration between the eyes without subfoveal nodule and eyes with subfoveal nodule were calculated by Mann-Whitney-Wilcoxon nonpaired test. The statistical significances for the disease stage, mean number of intravitreal anti–vascular endothelial growth factor (anti-VEGF) injection, mean number of focal/scatter LASER, and final best-corrected visual acuity (BCVA) between the eyes without subfoveal nodule and eyes with subfoveal nodule were calculated by Fisher exact test.
Results
Medical records of 44 patients with Coats disease were reviewed. At presentation, 21 patients (47.7%) presented stage 2A, 13 patients (29.5%) presented stage 2B, two patients (4.6%) presented stage 3A1, three patients (6.8%) presented stage 3A2, three patients (6.8%) presented stage 4, and two patients (4.6%) presented stage 5.
Among 21 patients presenting stage 2A, no patient showed subfoveal nodule or developed macular fibrosis. Among 13 patients presenting stage 2B, 10 patients (76.9%) showed subfoveal nodule and seven patients (70% among patients with subfoveal nodule) with subfoveal nodule developed macular fibrosis. Among two patients presented stage 3A1, one patient (50%) showed subfoveal nodule and developed macular fibrosis, while another one did not have sufficient images to identify the presence of subfoveal nodule or the development of macular fibrosis. A mong three patients presented stage 3A2, two patients (66.7%) showed subfoveal nodule and developed macular fibrosis, while another one did not. Among three patients presented stage 4, one patients (33.3%) showed subfoveal nodule and developed macular fibrosis, while other two did not have sufficient images to identify the presence of subfoveal nodule or the development of macular fibrosis. Two patients presented stage 5 did not have sufficient images to identify the presence of subfoveal nodule or the development of macular fibrosis.
In this study, we included patients with stage 2B, characterized by localized exudation in the foveal region causing subfoveal nodule, and stage 3A1, whether exudative retinal detachment does not involve the fovea. Thirty three patients were excluded of which 30 patients presented stage 2A or 3A2, 4, and 5, and two patients did not have follow-up visits. A total of 12 patients were finally enrolled, who were examined between October 2008 and July 2023. Images including fundus photo, OCT, and FA were available in all patients.
Among the remaining 12 patients presenting stage 2B or 3A1, nine patients (75.0%) presented with subfoveal nodule. Comparative analysis was done between the patients with subfoveal nodule and the patients without subfoveal nodule (Table 1). All 12 patients were male, and there were no significant differences in age, stage of the disease (2B and 3A1), extension of retinal exudation and peripheral telangiectasia, and follow-up duration between two groups. Fig. 2A–2D shows the cases of a patient with subfoveal nodule and a patient without subfoveal nodule. There were significantly more patients with severe visual loss (≤20 / 200) among the patients with subfoveal nodule (p = 0.045), and the cause for severe visual loss was macular fibrosis in all cases. No patient with subfoveal nodule showed final BCVA higher than 20 / 40 and one patient without subfoveal nodule showed final BCVA higher than 20 / 40. Macular fibrosis developed significantly more frequently in the patients with subfoveal nodule (p = 0.045).
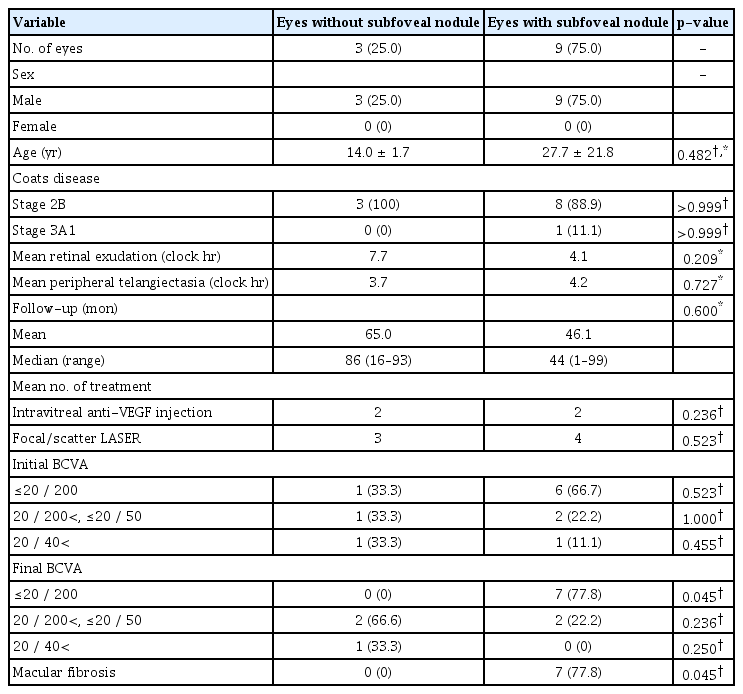
Comparative analysis between eyes without and with subfoveal nodule among patients with stage 2B or 3A1 Coats disease (n = 12)

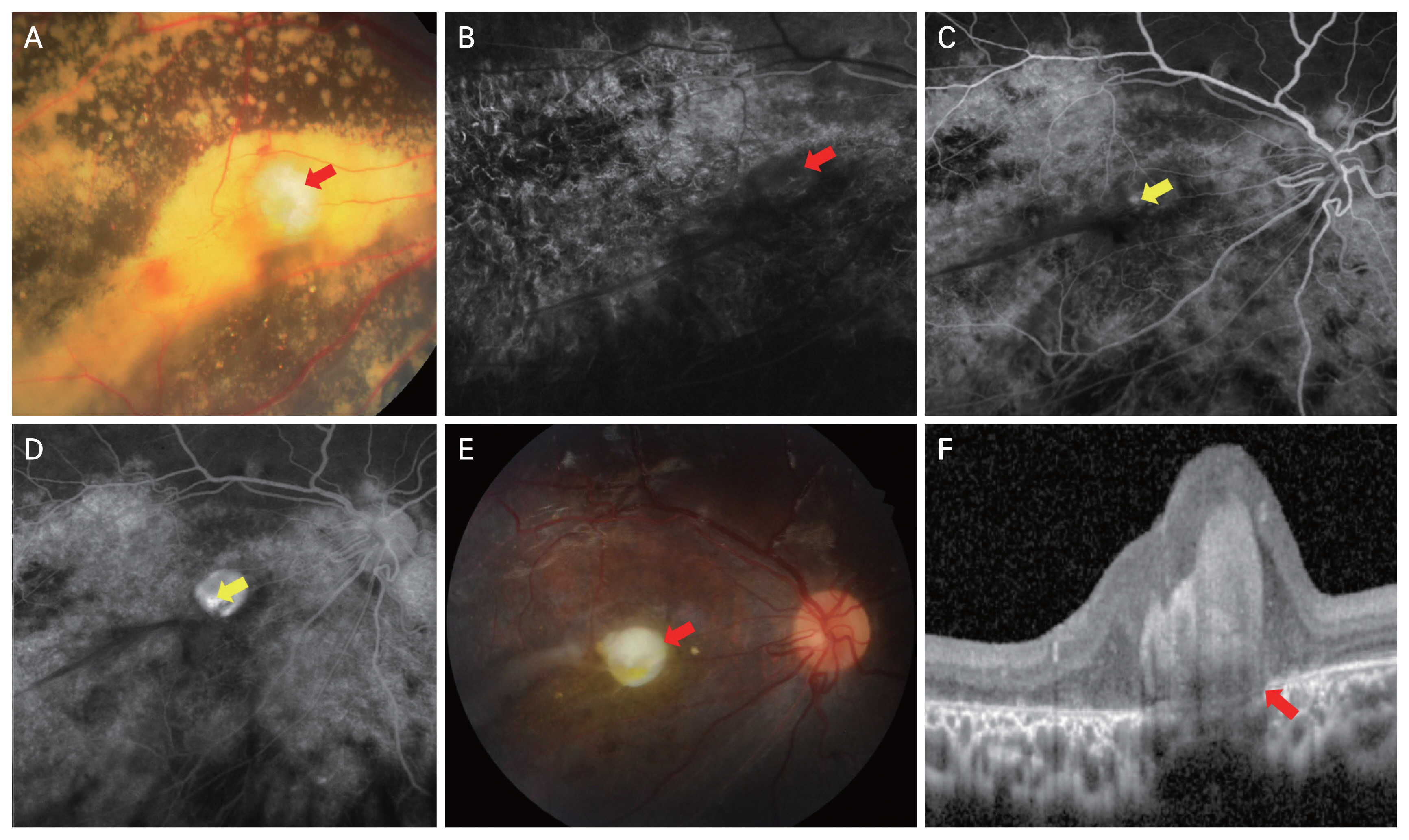
Cases of Coats disease with or without subfoveal nodule. (A, B) A 2-year-old male patient. (A) Color fundus photograph showed a yellow lesion suggestive of retinal exudates (white arrow) and subretinal nodule (red arrow). (B) Spectral-domain optical coherence tomography confirmed the presence of subretinal nodule (arrow). (C, D) A 15-year-old male patient. (C) On color fundus photograph, yellow exudates are visible around foveal area. (D) There is no subfoveal nodule confirmed in spectral-domain optical coherence tomography.
On the fundus examination, a distinctive vascular structure comprising a small artery and vein of intermediate size was identified as entering the subfoveal nodule. Eight patients who presented with this subfoveal nodule underwent FA. Among these, six patients underwent FA at the time of their initial diagnosis, while three patients had it performed on their last visit, following the development of macular fibrosis. In the early stages, FA revealed an area with diminished fluorescence that corresponded to the nodule. This region either retained its subdued brightness or exhibited a slight increase in brightness with minimal fluid leakage during the later stages, as depicted in Fig. 3A–3F. Following the onset of macular fibrosis, most cases displayed an escalating brightness pattern with well-defined borders, indicative of staining. Less frequently, there was an expanding and intensifying brightness corresponding to fluid leakage. Additionally, among eight patients with subfoveal nodule who underwent FA, there were visible connections between retinal blood vessels (retinal–retinal anastomosis) in four eyes (50.0%) and neovascularization in one eye (12.5%). At the last visit, SDOCT was conducted on eight patients with subfoveal nodules. The scans revealed a distinctly outlined, highly reflective lesion located in the subfoveal region, accompanied by a shadowing effect. There was also a noticeable disruption in the arrangement of retinal layers and an increase in thickness, characterized by an abrupt rise in the retinal layers due to the presence of the highly reflective nodule below (Fig. 4A–4E).
A 2-year old male patient presenting with stage 2B Coats disease. (A) Color fundus photograph shows a subfoveal nodule (arrow). (B–D) Fluorescein angiography. A hypofluorescent subfoveal lesion is shown during (B) the early phase (red arrow) and (C) mid-phase, which became hyperfluorescent with minimal leakage during (D) the late phase because of the presence of a retinal–retinal anastomosis (yellow arrows). After 4 years and 3 months of follow-up, subfoveal nodule has turned into macular fibrosis as found in (E) the fundus photograph (arrow) and (F) spectral-domain optical coherence tomography (arrow). Atrophic change of subfoveal nodule is identifiable when compared with Fig. 2B and disruption of outer segments, Ellipsoid zone, interdigitation zone, retinal pigment epithelium, and Bruch complex is found.
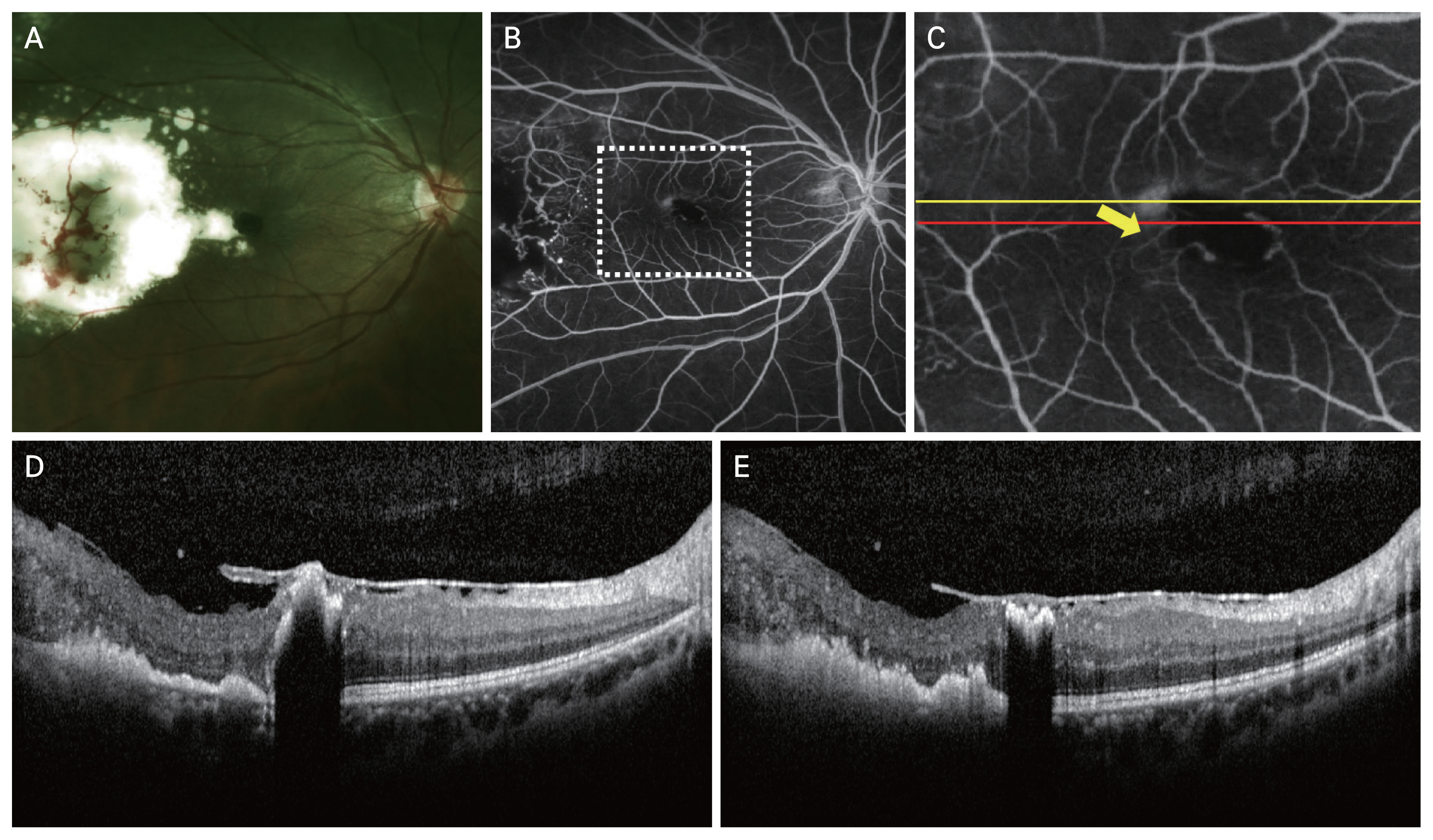
A 28-year-old male presenting with stage 2B Coats disease and a subfoveal nodule. (A) Color fundus photograph. (B) Fluorescein angiography showed a retinal–retinal anastomosis at the level of the subfoveal nodule (dotted rectangle). (C) Magnification of the rectangular area showing the retinal–retinal anastomosis (arrow). Spectral-domain optical coherence tomography images taken along (D) the yellow line of panel C and (E) the red line of panel C, which show the subfoveal nodule, hyper-reflective materials in the outer retinal layers corresponding to the hard exudates, and irregular retinal layers.
Discussion
Subfoveal nodule in Coats disease is a nodular aggregate of partially fibrinous material admixed with retinal pigment epithelium (RPE)-derived cells and macrophages [6]. Sometimes, the subfoveal nodule is misdiagnosed as retinoblastoma due to overlapping clinical features including the mass-like appearance, retinal abnormalities, retinal detachment or fluid accumulation, and imaging findings [2,6]. Coats disease presents as a unilateral disorder in older male children with no family history generally. On the other hand, retinoblastoma is more often bilateral with family history and no sex predilection. The presence of subretinal and intraretinal calcification suggests retinoblastoma strongly, while B-scan echography of subretinal and intraretinal spaces reveals no calcification in Coats disease. Advanced Coats disease often shows a closed or narrow funnel-shaped retinal detachment, retinal thickening and poor retinal mobility in A- and B-scan echography.
The subfoveal nodule in Coats disease can lead to a poor visual outcome for several reasons [3,5]. Firstly, subfoveal region is the central part of the retina responsible for central vision and therefore, any abnormality, including the nodule and further macular fibrosis, can directly interfere with central vision, leading to a significant loss of visual acuity. Secondly, the nodule itself can distort and damage the central retina, causing a localized loss of function. The presence of the nodule may lead to a disruption in the normal structure of the retinal layers and affect visual outcome. Thirdly, macular edema secondary to subfoveal nodules can lead to swelling and distortion of the macular tissue, further impairing central vision.
The macular fibrosis following the subfoveal nodule in certain cases can be induced by various factors [4,7]. The presence of telangiectatic blood vessels and leakage of fluid can lead to chronic inflammation within the retina. Inflammation can trigger the formation of fibrous tissue as part of the healing response. Secondly, in Coats disease, VEGF can be overexpressed, promote the formation of abnormal blood vessels and contribute to the development of fibrosis. Daruich et al. [5] also stated that the existence of subfoveal nodule at presentation can be a predictive factor for macular fibrosis development because of the subsequent development of macular fibrosis in patients with Coats disease. Thus, they suggested that it would be apposite for the classification of Coats disease to include the existence of subfoveal nodule.
In our retrospective review of medical records of 12 patients diagnosed with either stage 2B or 3A1 Coats disease, nine patients (75.0%) presented with a subfoveal nodule which advanced into macular fibrosis, and the progression was related to a decline in visual acuity levels. Conversely, the patients who initially displayed subfoveal exudation but lacked a subfoveal nodule experienced more favorable outcomes in terms of their final visual acuity.
The classification system for Coats disease, conceived by Shields et al. [2], takes into account the presence and location of lipid exudates, offering insights into the expected visual prognosis. This system distinguishes between the patients who do not have foveal exudation and those who do have foveal exudation as stages 2A and 2B, and typically, the patients with stage 2A Coats disease tend to achieve superior visual outcomes compared to the patients with stage 2B Coats disease. The study underscores the critical role of a subfoveal nodule as a predictive factor for a less favorable visual outcome, primarily due to the subsequent development of macular fibrosis.
However, it is essential to note that this predictive value is most applicable to stage 2B patients, as stage 3A1 patients may encounter other severe causes of visual impairment as their disease progresses, including tractional retinal detachment. In light of these findings, as previous studies imply [2,5,7], our study countenances the adjustment to the existing classification system to incorporate the presence of a subfoveal nodule, potentially enhancing the identification of patients with predisposition to the development of macular fibrosis and experiencing a poorer visual outcome.
Additionally, a consistent vascular characteristic linked with subfoveal nodules was revealed by FA. In most instances, this characteristic manifested as retinal–retinal anastomosis, although a few cases displayed neovascularization. Despite Coats disease primarily affecting the peripheral retinal vasculature, the existence of retinal–retinal anastomosis in the macular region suggests a broader spectrum of congenital retinal telangiectasis, which encompasses type 1 idiopathic macular telangiectasia [8].
Furthermore, recent findings revealed that patients with type 1 macular telangiectasia exhibited irregular connections among retinal capillaries in the perifoveal region [9]. However, there have been no instances of subfoveal nodules occurring independently of peripheral telangiectasia reported, making it impossible to conclusively establish that all subfoveal nodules originate without the influence of peripheral exudation. It is possible that certain cases may result from a significant buildup or leakage of substances in the macular region due to abnormalities in the peripheral blood vessels.
Another study by Sigler and Calzada [10] reported the presence of retinal angiomatous proliferation accompanied by chorioretinal anastomosis in 24% of 21 patients with Coats disease who were treated with intravitreal bevacizumab. The authors postulated that Coats’ disease might involve a developmental anomaly during the formation of the retinal midcapillary plexus, leading to type 3 neovascularization with chorioretinal anastomosis.
Due to the retrospective nature, the relatively small patient pool, the occasional unavailability of certain imaging techniques, and the limitation concerning the follow-up duration became the constraints of this study. There seems to be a tendency which was not proven to be statistically significant due to the small patient pool given the rarity of Coats’ disease, regarding the mean age of the patients without subfoveal nodule and the patients with subfoveal nodule. Furthermore, poor baseline visual acuity level could have affected final visual acuity level, even though the baseline visual acuity level did not show statistically significant difference between the eyes without subfoveal nodule and the eyes with subfoveal nodule. However, there are only some case studies regarding Coats’ disease in Korean, and there is no previous study including the analysis of subfoveal nodules in Korean patients with Coats disease. For advanced statistical analysis, a multicenter study with a larger patient pool would be desirable.
In our study, we compared the intraocular findings of Coats disease in Korean patients in accordance with the disease stage, and verified that the subfoveal nodule induces macular fibrosis and affects the visual outcome. The existence of a subfoveal nodule at the initial diagnosis serves as an indicator predicting the development of macular fibrosis and a less favorable visual outcome in the patients with Coats disease. Further studies toward the therapeutic approach for the subfoveal nodule and macular fibrosis are needed.
Acknowledgements
None.
Notes
Conflict of Interest
None.
Funding
This study was supported by the National Research Foundation of Korea (NRF) grant, funded by the Korean Ministry of Science and ICT (No. 2022R1A2C4002114).