 |
 |
| Korean J Ophthalmol > Volume 38(1); 2024 > Article |
|
Abstract
Purpose
The aim of this study is to investigate changes in intraocular pressure (IOP) and anterior-segment parameters before and after cataract surgery, vitrectomy, and combined surgery.
Methods
The records of patients who had undergone cataract surgery (cataract group), vitrectomy (vitrectomy group), or combined cataract surgery and vitrectomy (combined group) at our hospital were retrospectively examined. The vitrectomy group consisted of pseudophakic eyes. IOP and anterior-segment measurements, including anterior chamber depth (ACD), angle opening distance (AOD), trabecular-iris angle (TIA), and trabecular-iris space area (TISA), were measured using swept-source anterior-segment optical coherence tomography before and 6 months after surgery in 41, 15, and 40 eyes, respectively.
Results
In the cataract and combined groups, there was a decrease in IOP (cataract group: from 15.8 to 13.4 mmHg, p < 0.001; combined group: from 15.8 to 14.2 mmHg, p = 0.002) and an increase in the central corneal thickness after surgery (p < 0.001). The ACD increased in all groups, with a smaller increase in the vitrectomy group (p < 0.03). Postoperative AOD, TIA, and TISA were significantly increased in the cataract and combined groups (p < 0.02). Higher preoperative IOP and larger IOP reduction after surgery were correlated with smaller preoperative AOD, TISA, and TIA in cataract and combined groups (p < 0.034). A small preoperative ACD was related to smaller preoperative AOD, TISA, TIA (r > 0.649, p < 0.001), and postoperative IOP reduction in the cataract and combined groups (r = 0.377, p = 0.018 and r = 0.559, p = 0.001, respectively).
Conclusions
Compared to the vitrectomy group, the cataract and combined groups showed reduced postoperative IOP and increased AOD, TISA, and TIA. In these two groups, patients with shallower preoperative ACDs showed greater changes in IOP after surgery. Changes in IOP after surgery are thought to be related to changes in the anterior segment caused by the removal of the crystalline lens.
Many studies have been conducted on postoperative changes following cataract surgery as it is the most commonly performed ophthalmic surgery. It has been widely reported that the anterior chamber deepens, and intraocular pressure (IOP) decreases after cataract surgery. Several reports have shown that as the crystalline lens volume decreases, the anterior chamber deepens, and the anterior chamber angle increases in eyes that undergo cataract surgery [1,2]. However, the exact mechanism underlying reduced postoperative IOP has not yet been elucidated. The inflammatory effects of fibrosis, changes in the ciliary body, and anatomical changes in the anterior segments are believed to play important roles in this mechanism [3].
There are controversial results regarding postoperative changes in the IOP and anterior chamber angle following vitrectomy. Some reports have confirmed a significant increase in IOP after pars plana vitrectomy, whereas other studies have shown no significant postoperative changes in IOP [4-7]. Only a few studies have been conducted on changes in the anterior-segment structures after vitrectomy [8,9].
Various studies have been conducted to measure changes in the anterior segment after cataract surgery and vitrectomy, using slit-lamp examination, gonioscopy, ultrasound biomicroscopy (UBM), and anterior-segment optical coherence tomography (AS-OCT). Anterior-segment examination using AS-OCT showed similar results and accuracy to UBM. Recently, with the development of AS-OCT, more accurate analysis of the anterior-segment parameters has become possible [10,11].
To the best of our knowledge, there is no consensus regarding the changes in anterior-segment measurements before and after vitrectomy or phacovitrectomy. Thus, the present study aimed to compare changes and relationships between the IOP and anterior chamber parameters measured using swept-source AS-OCT in patients who had undergone cataract surgery, vitrectomy, or combined surgery.
This study was performed in accordance with the tenets of the Declaration of Helsinki and approved by the Institutional Review Board of Pusan National University Hospital (No. 05-2022-218). All patients were provided with written informed consent after they were apprised of the surgical procedure and their information.
The medical records of patients who underwent cataract surgery, vitrectomy, or combined surgery at Pusan National University Yangsan Hospital (Yangsan, Korea) between January 2021 and August 2022 and were followed up for more than 6 months were retrospectively reviewed and consecutively enrolled.
Patients were categorized into the cataract surgery group (cataract group), simple vitrectomy group (vitrectomy group), and combined cataract surgery and vitrectomy (combined group), according to the surgical procedure performed on each patient. The vitrectomy group included all the pseudophakic eyes. To exclude the influence of underlying factors on IOP, vitrectomy was performed only in patients with an epiretinal membrane (ERM) and macular hole (MH), and cataract surgery was performed only in patients without vascular proliferative complications. ERM was defined as a thin membrane covering the front of the retina observed on OCT and fundus, and MH was defined as a full-thickness central foveal hole observed on fundoscopy with a defect of the entire retinal layer confirmed on OCT. The exclusion criteria were as follows: (1) presence of ocular diseases other than ERM and MH in the combined and vitrectomy groups; (2) history of ocular trauma; (3) high myopia (spherical equivalent ≥ −6.0 diopters or axial length ≥26 mm); (4) ocular conditions such as retinal detachment and intraocular inflammation that may affect IOP; (5) patients with glaucoma or preoperative ocular hypertension (IOP >21 mmHg); (6) patients with ocular neovascularization including ischemic retinal vein occlusion and proliferative diabetic retinopathy, except for age-related macular degeneration; (7) patients with corneal opacity, keratoconus, keratopathy, or keratitis within the last 6 months; (8) patients with other media opacities; and (9) silicone oil used during vitrectomy.
In the cataract group, phacoemulsification was performed using the Infinity Vision System (Alcon Laboratories Inc) with a 19-gauge phacoemulsification tip. After implantation of the intraocular lens (IOL), the viscoelastics were removed by placing an irrigation and aspiration cannula under the IOL. A 25-gauge three-port pars plana vitrectomy was performed by a single surgeon (SML) using the Constellation Vision System (Alcon Laboratories Inc). The vitreous was removed as much as possible from the ora serrata, and assistance with the indentation technique was used if visualization of the ora serrata was not possible. In some cases, room air or 18% sulfur hexafluoride was used for tamponade when needed. In the combined group, phacoemulsification was performed in phakic patients with an age of 50 years or more or with a preoperative nuclear opalescence grade of three or higher on the Lens Opacities Classification System III [12]. The surgical method was the same as that used for the cataract group and was performed before vitrectomy.
All patients underwent a comprehensive ophthalmologic examination, including measurement of the best-corrected visual acuity (BCVA), IOP with a noncontact tonometer (TX-20, Canon), refractive error converted to the spherical equivalent, fundus examination, axial length using partial coherence laser interferometry (IOLMaster 500, Carl Zeiss Meditec AG), and anterior-segment parameters using swept-source AS-OCT (CASIA2, Tomey Corp) at the initial visit. IOP and AS-OCT were assessed at each follow-up visit at 1, 3, and 6 months.
Anterior-segment parameters, including the central corneal thickness (CCT), anterior chamber depth (ACD), lens thickness (LT), angle-to-angle distance (ATA), lens vault (LV), and anterior chamber angle-associated parameters at 500 and 750 μm were measured by AS-OCT. The angle opening distance (AOD), trabecular-iris space area (TISA), and trabecular-iris angle (TIA) were defined as the anterior chamber angle-associated parameters. The analyzed parameters are described in Fig. 1A-1G and calculated by using embedded automated algorithms in the swept-source OCT [13,14]. Each of parameters was defined as follows [13,14]: ACD is the maximum distance between the corneal endothelium and the anterior surface of the lens; LV is the distance between the horizontal line passing the anterior pole of the crystalline lens and the horizontal line joining the two scleral spurs; ATA is the distance between the angle recesses; AOD is the length of the line perpendicular to the cornea between the iris and the cornea; TISA is the trapezoidal area with anterior border of the AOD line, posterior border of a line perpendicular to the inner scleral wall to the opposing iris at the scleral spur, superior border of corneoscleral wall, and inferior border of the iris surface; and TIA is the angle at the apex of a triangle with the angle recess as the vertex and the AOD line as the base. AOD, TISA and TIA were measured at distances of 500 and 750 μm from the scleral spur.
The values of each parameter were obtained automatically using an embedded program on the device. If the contour of the structures was ambiguous and could not be measured automatically, the location of the main structures was manually specified on a zoomed-in image magnified by more than 200%. The AOD, TISA, and TIA were averaged from the nasal and temporal values obtained in the horizontal direction.
The BCVA measured using the Snellen chart was converted to the logarithm of the minimum angle of resolution (logMAR) scale. Sex and the laterality of the eyes were compared using the chi-square test, and continuous variables such as the BCVA, refractive error, axial length, IOP, and anterior-segment parameters were compared among the three groups using a one-way analysis of variance. Scheffé test was used for post hoc test. A paired t-test was used for preoperative and postoperative comparisons of continuous variables in each group, and Pearson correlation analysis was performed to determine the correlation between each factor. Multiple regression analysis was performed on ACD, LT, and LV to identify factors affecting the anterior chamber angle-associated parameters at 750 μm. In the multiple regression analysis, if the tolerance was less than 0.1 or the variance inflation factor was greater than 10, multicollinearity was considered, and the items with multicollinearity were removed. Multiple regression analyses were performed using both forward and backward selection, and the method that provided the most appropriate results was selected. Statistical analyses were performed using IBM SPSS ver. 23.0 (IBM Corp). A p-value of <0.05 was considered statistically significant.
There were 41, 15, and 40 eyes in the cataract, vitrectomy, and combined groups, respectively. Regarding baseline characteristics (Table 1), there was no significant difference between groups, except for age. The cataract group was older than the combined group (post hoc Scheffé test, p < 0.019). There was a significant decrease in IOP at 1 and 6 months from 15.8 ± 1.8 to 13.7 ± 2.3 and 13.4 ± 2.0 mmHg, respectively, in the cataract group and from 15.8 ± 3.3 to 14.4 ± 3.3 and 14.2 ± 2.5 mmHg, respectively in the combined group (p < 0.001, p = 0.026, p = 0.002, respectively).
CCT increased at 1 and 6 months after surgery compared to baseline in the cataract and combined groups (p < 0.001) (Table 2 and Fig. 2A-2C). However, CCT was decreased at 6 months compared to that at 1 month in both groups ( p < 0.001 and p = 0.003). ACD increased at 6 months in all groups (p < 0.030) (Table 2 and Fig. 2). The increase in the vitrectomy group was relatively small compared with that in the other two groups (post hoc Scheffé test: vitrectomy group vs. cataract group, p = 0.020; vitrectomy vs. combined group, p = 0.028). ATA showed no significant changes in any of the groups. LV moved backward at 6 months in the cataract and combined groups (p < 0.001) (Table 2). AOD, TISA, and TIA increased compared to baseline in both the cataract and combined groups (p < 0.02) (Table 2 and Fig. 3A-3C).
The preoperative parameters of preoperative ACD, AOD at 500 and 750 μm, TISA at 500 and 750 μm, and TIA at 500 and 750 μm in the vitrectomy group were significantly higher than those in the cataract and/or combined groups ( p < 0.001, p <0.001, p = 0.003, p = 0.010, p = 0.047, p = 0.002, p = 0.013, and p = 0.017, respectively) (Table 2), whereas the preoperative LV of the vitrectomy group was significantly lower (p < 0.001). Preoperative IOP and CCT showed no significant differences among the three groups.
In the cataract and combined groups, IOP reduction during 6 months was correlated with a smaller preoperative AOD, TISA (except for TISA at 500 μm of the cataract group), and TIA (p <0.038) (Table 3). There was no correlation between IOP changes and changes of the anterior chamber angle-associated parameters. Preoperative IOP of cataract and combined groups was negatively correlated with preoperative AOD, TISA (except for TISA at 500 μm in the cataract group), and TIA (except for TIA at 500 μm in the combined group) (−0.478 < r < −0.336, p < 0.037). Postoperative IOP had no correlations with the postoperative AOD, TISA and TIA in all groups. A small preoperative ACD was related to postoperative IOP reduction in the cataract and combined groups at 6 months (r = 0.377, p = 0.018 and r = 0.559 and p = 0.001, respectively).
Preoperative LV was correlated with preoperative AOD, TISA (except for TISA at 500 μm), and TIA (r < −0.552, p < 0.003), and LV at 6 months was correlated with changes in AOD, TISA, and TIA (r > 0.534, p < 0.004) in the cataract group.
Preoperative LT was related to preoperative AOD, TISA, and TIA, and changes of AOD, TISA, and TIA in the combined group (r < −0.682, p < 0.001 and r > 0.492, p < 0.004, respectively).
The preoperative ACD in the cataract and combined groups was associated with preoperative AOD, TISA (except for TISA at 500 μm of the cataract group), and TIA (r > 0.649, p < 0.001 and r > 0.791, p < 0.001, respectively). In addition, in the cataract and combined groups, there were significant correlations between the preoperative ACD and LT (cataract group: r = −0.708, p < 0.001; combined group: r = −0.808, p < 0.001), preoperative LV and LT (cataract group: r = 0.673, p < 0.001; combined group: r = 0.713, p < 0.001), and preoperative ACD and LV (cataract group: r = −0.808, p < 0.001; combined group: r = −0.799, p<0.001) (Table 4).
Multiple regression analysis showed that preoperative ACD was the most relevant factor determining preoperative AOD, TISA, and TIA at 750 μm in the cataract group (standardized coefficient β = 0.863, p < 0.001; β = 1.009, p < 0.001; β = 1.094, p < 0.001, respectively) and combined group (β = 0.865, p < 0.001; β = 0.836, p < 0.001; β = 0.570, p = 0.002, respectively) with LT being an additional relevant factor in the cataract group (β = 0.505, p = 0.004; β = 0.455, p = 0.021; β = 0.638, p = 0.001, respectively). Regarding postoperative AOD, TISA, and TIA at 750 μm, postoperative ACD was identified as a relevant factor only in the combined group (β = 0.647, p = 0.002; β = 0.436, p = 0.040; β = 0.685, p = 0.002, respectively). Associations between the changes in AOD, TISA, and TIA, and changes in ACD and LV were not significant.
IOP reduction after cataract surgery, including extracapsular cataract extraction and phacoemulsification, has been demonstrated in various articles [15-17]. However, few studies have been conducted on IOP changes after a combined operation, and controversies regarding IOP changes after vitrectomy had existed [18,19]. Masis Solano and Lin [3] reviewed the pathophysiological mechanisms of IOP reduction after cataract extraction using molecular theory, which demonstrates the action of cytokines or prostaglandins on the trabecular meshwork, physiological theory by the position changes of the ciliary body, and biomechanical theory according to changes in the anterior-segment anatomy. Recently, with the development of AS-OCT, interest in anterior-segment image analysis has increased, and the number of studies analyzing changes in the anterior-segment anatomy after cataract surgery using various parameters with AS-OCT has increased. Only a few studies have investigated the effects of vitrectomy or phacovitrectomy on anterior-segment parameters using AS-OCT [8]. Thus, we aimed to analyze and compare changes in the anterior-segment anatomy and IOP not only after simple cataract surgery but also after vitrectomy or combined surgery and to determine the relationship between the anterior-segment parameters and IOP.
In the present study, changes in the IOP and anterior chamber parameters associated with cataract surgery, simple vitrectomy, and combined surgery were investigated. Postoperative IOP decreased in the cataract and combined groups but not in the vitrectomy group. After surgery, CCT and ACD increased, and LV moved backward in the cataract and combined groups. The anterior chamber angle-associated parameters, including AOD, TISA, and TIA at 500 and 750 μm in the cataract and combined groups increased after surgery, but there was no significant change in the vitrectomy group. When preoperative measurements of the anterior chamber angle-associated parameters and ACD were smaller, preoperative IOP was higher was greater in the cataract and combined groups. IOP changes was negatively related with preoperative ACD in the cataract and combined groups. Postoperative IOP was not correlated with postoperative anterior chamber angle-associated parameters. In the regression analysis, preoperative ACD was found to be related to the preoperative anterior chamber angle-associated parameters in both groups. There were no common factors related to the postoperative parameters or changes in the parameters in both groups. In the vitrectomy group, no significant changes in the anterior-segment measurements were observed, except for a relatively small changes in ACD compared with the other groups.
Yang et al. [19] and Hirasawa et al. [20] reported that IOP decreased from 13.5 ± 2.9 to 11.9 ± 2.8 mmHg and from 16.5 ± 4.0 to 12.6 ± 2.8 mmHg, respectively, after cataract surgery. In our study, a similar significant decrease in IOP was observed in the cataract group after surgery. In terms of vitrectomy, Wu et al. [7] reported that the rate of eyes with elevated IOP after surgery was 19.2%, which was significantly higher than 4.5% in the contralateral eye, while the mean IOP decreased from 15 ± 3.5 to 14.7 ± 2.8 mmHg. In a study by Cabuk and Cekic [21], IOP was found to be consistently higher than the baseline value during the 12 months of follow-up after vitrectomy or phacovitrectomy. In the research of Mi and Thompson [22], the mean IOP showed no significant change after vitrectomy regardless of lens status. Yamamoto et al. [23] concluded that at 12 months after vitrectomy or phacovitrectomy, changes in IOP was not significant in ERM and MH, but IOP significantly increased in rhegmatogenous retinal detachment. Unlike other studies that did not consider lens status, ocular disease affecting IOP, or combining cataract surgery, our study only enrolled pseudophakic patients with ERM and MH in the vitrectomy group and the effect of vitrectomy on the IOP, which showed no significant changes, could be more accurately confirmed. Regarding the combined surgery, Ki-I et al. [18] reported a decrease in IOP at 3 months, which then returned to baseline at 12 months, and the result of Byun et al. [24] showed no significant IOP changes until 12 months after surgery. In our study, the IOP continuously decreased for 6 months, which is different from the findings of the above studies. In the report by Byun et al. [24], diseases with a risk of glaucoma or the tendency of postoperative IOP increase was included, and it is presumed that these factors affected the results. Compared to the results of Ki-I et al. [18], IOP did not show a tendency of rebounding to the baseline values after 3 month in our study. Because there was a decrease in IOP after cataract surgery and no significant change in IOP after vitrectomy, the decrease in IOP after combined surgery was considered a reasonable result.
Regarding changes in the anterior-segment parameters after cataract surgery, Yang et al. [19] reported that ACD increased from 2.60 ± 0.35 to 3.43 ± 0.35 mm, and AOD 500 increased from 0.26 ± 0.03 to 0.44 ± 0.04 after cataract surgery. Kim et al. [25] reported similar significant postoperative increases in TIA, AOD, and TISA. These results are consistent with the current study. Regarding the vitrectomy, in a study by Khodabande et al. [8], there were no changes in ACD, AOD, TISA, or TIA after vitrectomy. However, in a study by Toklu et al. [26], postoperative ACD was reduced in cases of complete vitrectomy with scleral indentation. They suggests that the presence of the anterior vitreous can affect ACD after vitrectomy. Unlike previous studies, the results of our study showed an increase in ACD, and to confirm these differences, it is necessary to check the additional confirmation of the relationship between the extent of anterior vitrectomy and ACD. For the combined surgery, no studies have been reported on anterior-segment parameters using AS-OCT, except for ACD. According to the report of Seo et al. [27] there were larger increase of ACD in the combined surgery group than that in cataract surgery group and decrease of ACD in vitrectomy group. Those results were different from our result which showed no difference in ACD between the cataract and combined surgery groups and increase of ACD in vitrectomy group. The difference between two studies in the vitrectomy group is that our study targeted pseudophakic eyes, but the study of Seo et al. [27] targeted phakic eyes in the vitrectomy group, so it is thought that the presence of the crystalline lens or cataract changes may have contributed to the difference. The exact cause of the difference in the results of the two studies regarding the additional ACD increase in the combined operation is unknown, but it may be due to differences in the extent of vitrectomy resection or used the IOL [26].
Regarding the anterior-segment parameters affecting IOP changes, Yang et al. [19] concluded that preoperative ACD, AOD, and changes in ACD and AOD were significant factors, and Perez et al. [28] concluded that ACD and AOD at 750 μm were related to IOP. Unlike the study by Yang et al. [19], changes in IOP in the result of our study and Perez et al. [28] were not associated with changes of anterior chamber parameters. This difference is thought to be due to the relatively small number of eyes included or changes in correlation between IOP and the anterior-segment parameters after surgery. In our result, since IOP had no correlation with AOD, TISA, and TIA after surgery, changes in IOP were not correlated with changes of AOD, TISA and TIA. These changes may occur because the effect of lens vaulting on preoperative IOP decreased after surgery and the influence of angle-related effects was reduced.
Increase of CCT after cataract surgery was assumed to be due to corneal edema and surgically induced astigmatism [29,30]. It is reported that the effect of corneal edema mostly improves in about 1 week and becomes similar to the preoperative level in approximately 3 to 12 months [30-32]. The effects of surgically induced astigmatism are reported to improve within 2 months [29]. In this study, the CCT improved close to baseline at 6 months, but the remaining small increase in thickness may be due to differences in surgical methods using superior cornea incision, relatively higher age than previous studies, differences in underlying diseases, and whether or not vitrectomy was used. Since IOP measurement is affected by CCT, IOP may be measured higher as corneal edema increases [20,33]. The increase of CCT at 1 month was approximately 20 μm, which may lead to underestimation of the IOP reduction. However, because the CCT difference between baseline and 6 months was less than 10 μm, it is thought that there was no significant effect on the IOP measurement at 6 months.
Our study identified factors influencing preoperative AOD, TISA, and TIA as LV and ACD in the cataract group and LT and ACD in the combined group. Preoperative ACD was identified as the most important factor in multiple regression analysis. Since LT, LV, and ACD are factors related to each other, not only in our study but also in many other articles, it is presumed that change in IOP after surgery in the cataract and combined groups are related to the anterior angle changes identified by AOD, TISA, and TIA according to changes in anterior-segment shape and ACD due to crystalline lens removal.
This study had several limitations. First, the number of eyes included in the study was small, particularly in the vitrectomy group. However, the data in the vitrectomy group were relatively constant and normally distributed in the normality test. Second, we did not analyze the axial length to determine the relationship between the overall eye shape and ACD. Third, we used a noncontact tonometer to measure IOP, which can be relatively inaccurate. To compensate for this, we obtained at least three measurements and rechecked them with a rebound-type tonometer to determine whether they differed from the previous measurement. Fourth, the total follow-up period for each participant was relatively short. Fifth, our vitrectomy group consisted with pseudophakic eyes and it did not reflect the results of vitrectomy for phakic eyes.
In conclusion, this study confirmed that decreased IOP and increased AOD, TISA, and TIA after cataract and combined surgery using AS-OCT. In addition, the preoperative AOD, TISA, and TIA were associated with changes in the IOP. The preoperative ACD was most related to changes in the anterior chamber angle after cataract and combined surgery. After vitrectomy surgery, there was a relatively small change in the ACD compared with that in the other surgeries and no significant change in the anterior-segment measurements was observed. Considering the correlation between lens vaulting and anterior chamber angle-related parameters, postoperative IOP changes are thought to be due to changes in the anterior angle according to crystalline lens removal.
References
1. Pereira FA, Cronemberger S. Ultrasound biomicroscopic study of anterior segment changes after phacoemulsification and foldable intraocular lens implantation. Ophthalmology 2003;110:1799-806.


2. Nonaka A, Kondo T, Kikuchi M, et al. Angle widening and alteration of ciliary process configuration after cataract surgery for primary angle closure. Ophthalmology 2006;113:437-41.


3. Masis Solano M, Lin SC. Cataract, phacoemulsification and intraocular pressure: is the anterior segment anatomy the missing piece of the puzzle? Prog Retin Eye Res 2018;64:77-83.


4. Framme C, Klotz S, Wolf-Schnurrbusch UE, et al. Intraocular pressure changes following 20G pars-plana vitrectomy. Acta Ophthalmol 2012;90:744-9.


5. Desai UR, Alhalel AA, Schiffman RM, et al. Intraocular pressure elevation after simple pars plana vitrectomy. Ophthalmology 1997;104:781-6.


6. Miele A, Govetto A, Fumagalli C, et al. Ocular hypertension and glaucoma following vitrectomy: a systematic review. Retina 2018;38:883-90.

7. Wu L, Berrocal MH, Rodriguez FJ, et al. Intraocular pressure elevation after uncomplicated pars plana vitrectomy: results of the Pan American Collaborative Retina Study Group. Retina 2014;34:1985-9.

8. Khodabande A, Mohammadi M, Riazi-Esfahani H, et al. Changes in anterior segment optical coherence tomography following pars plana vitrectomy without tamponade. Int J Retina Vitreous -2021. 7:15.




9. Ghomi Z, Ghassemi F. Changes in anterior segment parameters following pars plana vitrectomy measured by ultrasound biomicroscopy (UBM). Med Hypothesis Discov Innov Ophthalmol 2017;6:14-8.


10. Dada T, Sihota R, Gadia R, et al. Comparison of anterior segment optical coherence tomography and ultrasound bio-microscopy for assessment of the anterior segment. J Cataract Refract Surg 2007;33:837-40.


11. Radhakrishnan S, Goldsmith J, Huang D, et al. Comparison of optical coherence tomography and ultrasound biomicroscopy for detection of narrow anterior chamber angles. Arch Ophthalmol 2005;123:1053-9.


12. Chylack LT Jr, Wolfe JK, Singer DM, et al. The Lens Opacities Classification System III the Longitudinal Study of Cataract Study Group. Arch Ophthalmol 1993;111:831-6.


13. Chen X, Wang X, Tang Y, et al. Optical coherence tomography analysis of anterior segment parameters before and after laser peripheral iridotomy in primary angle-closure suspects by using CASIA2. BMC Ophthalmol 2022;22:144.




14. Triolo G, Barboni P, Savini G, et al. The use of anterior-segment optical-coherence tomography for the assessment of the iridocorneal angle and its alterations: update and current evidence. J Clin Med 2021;10:231.



15. Sengupta S, Venkatesh R, Krishnamurthy P, et al. Intraocular pressure reduction after phacoemulsification versus manual small-incision cataract surgery: a randomized controlled trial. Ophthalmology 2016;123:1695-703.


16. Zetterstrom C, Behndig A, Kugelberg M, et al. Changes in intraocular pressure after cataract surgery: analysis of the Swedish National Cataract Register Data. J Cataract Refract Surg 2015;41:1725-9.


17. Coh P, Moghimi S, Chen RI, et al. Lens position parameters as predictors of intraocular pressure reduction after cataract surgery in glaucomatous versus nonglaucomatous eyes. Invest Ophthalmol Vis Sci 2016;57:2593-9.



18. Ki-I Y, Yamashita T, Uemura A, Sakamoto T. Long-term intraocular pressure changes after combined phacoemulsification, intraocular lens implantation, and vitrectomy. Jpn J Ophthalmol 2013;57:57-62.



19. Yang HS, Lee J, Choi S. Ocular biometric parameters associated with intraocular pressure reduction after cataract surgery in normal eyes. Am J Ophthalmol 2013;156:89-94.


20. Hirasawa K, Nakakura S, Nakao Y, et al. Changes in corneal biomechanics and intraocular pressure following cataract surgery. Am J Ophthalmol 2018;195:26-35.


21. Cabuk KS, Cekic O. Intraocular pressure change and sustained intraocular pressure elevation after pars plana vitrectomy. Beyoglu Eye J 2021;6:96-101.


22. Mi CW, Thompson JT. Long-term follow-up of intraocular pressure after vitrectomy in eyes without preexisting glaucoma. Retina 2015;35:2543-51.


23. Yamamoto K, Iwase T, Terasaki H. Long-term changes in intraocular pressure after vitrectomy for rhegmatogenous retinal detachment, epi-retinal membrane, or macular hole. PLoS One 2016;11:e0167303.



24. Byun ZY, Lee JH, Lee SM, Hwang DD. Long-term analysis of surgically induced astigmatism after combined vitrectomy and cataract surgery versus cataract surgery alone. J Korean Ophthalmol Soc 2021;62:1029-35.


25. Kim M, Park KH, Kim TW, Kim DM. Changes in anterior chamber configuration after cataract surgery as measured by anterior segment optical coherence tomography. Korean J Ophthalmol 2011;25:77-83.



26. Toklu E, Altinisik M, Elbay A, Koytak A. Comparison of postoperative anterior segment changes associated with pars plana vitrectomy with and without vitreous base shaving. Int J Ophthalmol 2020;13:1745-52.



27. Seo S, Seong MC, Lim HW, et al. The changes of anterior chamber depth, axial length, refractive errors after combined vitrectomy. J Korean Ophthalmol Soc 2013;54:1032-7.

28. Perez CI, Chansangpetch S, Nguyen A, et al. How to predict intraocular pressure reduction after cataract surgery?: a prospective study. Curr Eye Res 2019;44:623-31.


29. Woo SJ, Lee JH. Effect of central corneal thickness on surgically induced astigmatism in cataract surgery. J Cataract Refract Surg 2003;29:2401-6.


30. Salvi SM, Soong TK, Kumar BV, Hawksworth NR. Central corneal thickness changes after phacoemulsification cataract surgery. J Cataract Refract Surg 2007;33:1426-8.


31. Aribaba OT, Adenekan OA, Onakoya AO, et al. Central corneal thickness changes following manual small incision cataract surgery. Clin Ophthalmol 2015;9:151-5.



Fig. 1
Representative case showing anterior-segment parameters measured by anterior-segment optical coherence tomography using manufacturer-provided software. (A,B) Angle opening distance (AOD) is the length of the line perpendicular to the cornea from the cornea to the iris. (C,D) Trabecular-iris space area (TISA) is marked as red trapezoidal area between the AOD line and a perpendicular line at the scleral spur (SS). (E,F) For trabecular-iris angle (TIA), the angle is measured from the angle recess (AR), which is the apex of a triangle with the AOD line as its base. AOD, TISA, and TIA are measured at (A,C,E) 500 μm and (B,D,F) 750 μm from the SS. (G) Central corneal thickness (CCT), anterior chamber depth (ACD), lens vault (LV), and angle-to-angle distance (ATA) are displayed on the cross-sectional image of anterior segment.
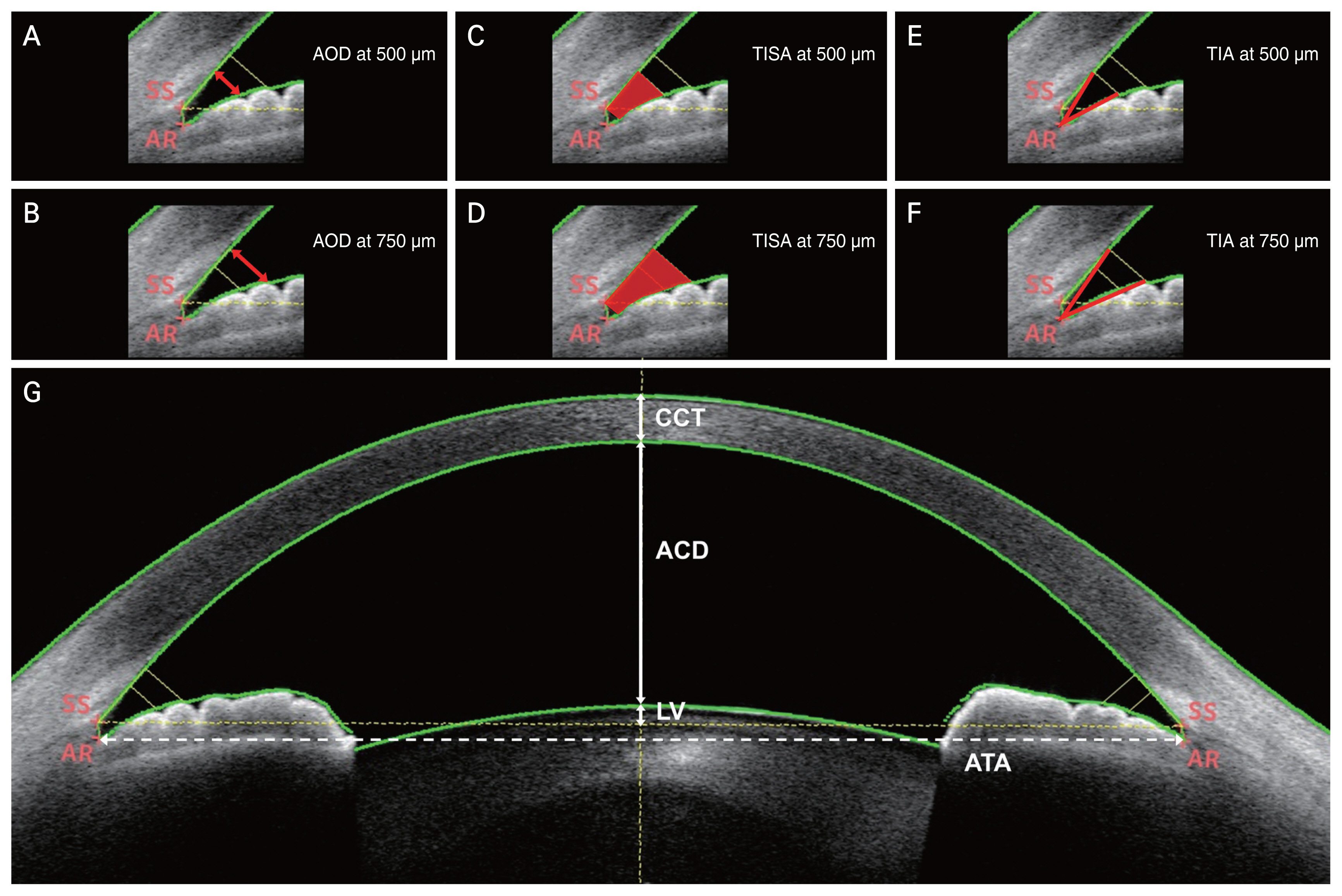
Fig. 2
Trends in (A) intraocular pressure (IOP), (B) central corneal thickness (CCT), and (C) anterior chamber depth (ACD) at baseline and 1 and 6 months after cataract surgery (cataract group), vitrectomy (vitrectomy group), or combined cataract surgery and vitrectomy (combined group). (A) In the cataract and combined groups, IOP significantly decreased 1 and 6 months postoperatively compared to baseline. (B) Similarly, the CCT significantly increased in these two groups while decreasing from postoperative 1 to 6 months. (C) In the case of ACD, all three groups showed a significant increase from baseline to 6 months postoperatively, while only the cataract and combined groups showed a significant increase from baseline to 1 month postoperatively.
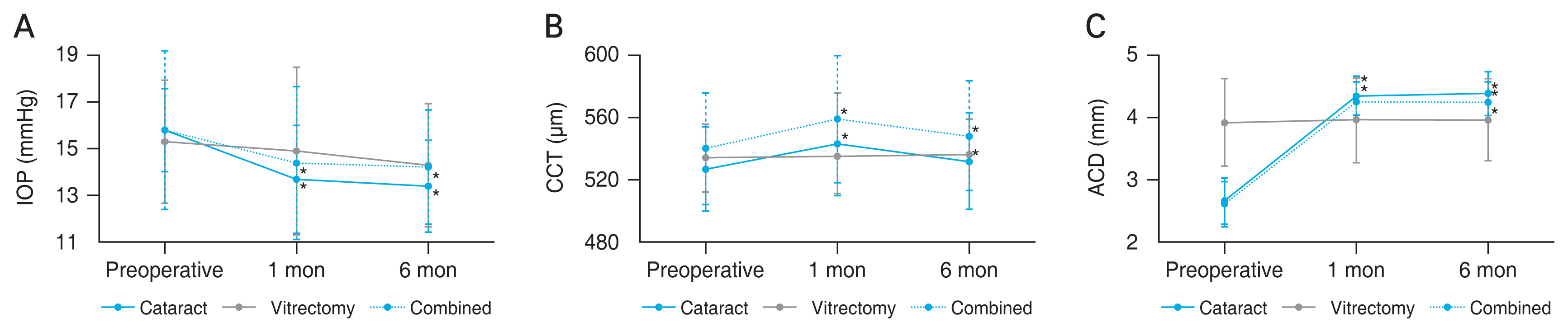
Fig. 3
Trends in (A) the angle opening distance (AOD) at 750 μm, (B) the trabecular-iris space area (TISA) at 750 μm, and (C) the trabecular-iris angle (TIA) at 750 μm at baseline and 1 and 6 months after surgery. AOD, TISA, and TIA at 750 μm showed similar changes from baseline to 6 months postoperatively, showing a significant increase from baseline to 1 month postoperatively and baseline to 6 months postoperatively in the cataract surgery and combined surgery groups.

Table 1
Baseline characteristics (n = 96)
| Characteristic | Cataract group (n = 41) | Vitrectomy group* (n = 15) | Combined group (n = 40) | p-value† |
|---|---|---|---|---|
| Sex | 0.259 | |||
| Male | 20 | 10 | 26 | |
| Female | 21 | 5 | 14 | |
| Age (yr) | 70.5 ± 10.0 | 67.5 ± 12.1 | 64.6 ± 7.9 | 0.023‡ |
| Laterality | 0.763 | |||
| Right eye | 23 | 7 | 23 | |
| Left eye | 18 | 8 | 17 | |
| BCVA (logMAR) | 0.70 ± 0.53 | 0.68 ± 0.43 | 0.61 ± 0.47 | 0.726 |
| Refractive error (spherical equivalent) | −0.53 ± 1.97 | 0.29 ± 1.09 | −0.01 ± 1.79 | 0.314 |
| Axial length (mm) | 23.8 ± 1.1 | 23.4 ± 1.1 | 23.5 ± 1.02 | 0.406 |
| Intraocular pressure (mmHg) | 15.8 ± 1.8 | 15.3 ± 2.6 | 15.8 ± 3.4 | 0.844 |
| Lens thickness (mm) | 4.6 ± 0.3 | - | 4.6 ± 0.3 | 0.871 |
Table 2
Intraocular pressure and anterior chamber parameters changes
| Parameter | Cataract group (n = 41) | Vitrectomy group (n = 15) | Combined group (n = 40) | p-value* | p-value (post hoc test†) | ||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Cataract vs. vitrectomy | Cataract vs. combined | Vitrectomy vs. combined | |||||
| Intraocular pressure (mmHg) | |||||||
| Preoperation | 15.8 ± 1.8 | 15.4 ± 2.6 | 15.8 ± 3.3 | 0.879 | 0.818 | 0.969 | 0.904 |
| 6 mon | 13.4 ± 2.0‡ | 14.3 ± 2.6 | 14.2 ± 2.5‡ | 0.267 | 0.890 | 0.943 | 0.974 |
| Central corneal thickness (μm) | |||||||
| Preoperation | 526 ± 27 | 534 ± 22 | 540 ± 36 | 0.147 | 0.770 | 0.147 | 0.778 |
| 6 mon | 532 ± 31‡ | 536 ± 23 | 548 ± 36‡ | 0.090 | 0.932 | 0.097 | 0.516 |
| Anterior chamber depth (mm) | |||||||
| Preoperation | 2.659 ± 0.376 | 3.920 ±0.694 | 2.606 ± 0.365 | 0.000 | <0.001 | 0.864 | <0.001 |
| 6 mon | 4.378 ± 0.359‡ | 3.958 ±0.677‡ | 4.240 ± 0.315‡ | 0.006 | 0.006 | 0.380 | 0.049 |
| Lens vault (μm) | |||||||
| Preoperation | 0.338 ± 0.285 | −0.910 ± 0.446 | 0.391 ± 0.266 | 0.000 | <0.001 | 0.797 | <0.001 |
| 6 mon | −1.281 ± 0.245‡ | −0.976 ± 0.268 | −1.184 ± 0.175‡ | 0.000 | 0.001 | 0.254 | 0.032 |
| AOD at 500 μm (mm) | |||||||
| Preoperation | 0.323 ± 0.126 | 0.496 ± 0.226 | 0.384 ± 0.167 | 0.003 | 0.004 | 0.252 | 0.050 |
| 6 mon | 0.482 ± 0.108‡ | 0.501 ± 0.216 | 0.542 ± 0.132‡ | 0.175 | 0.912 | 0.181 | 0.657 |
| AOD at 750 μm (mm) | |||||||
| Preoperation | 0.465 ± 0.186 | 0.691 ± 0.322 | 0.558 ± 0.253 | 0.010 | 0.012 | 0.230 | 0.208 |
| 6 mon | 0.720 ± 0.149‡ | 0.720 ± 0.325 | 0.770 ± 0.170‡ | 0.486 | >0.999 | 0.531 | 0.726 |
| TISA at 500 μm (mm2) | |||||||
| Preoperation | 0.130 ± 0.076 | 0.183 ± 0.073 | 0.141 ± 0.056 | 0.047 | 0.049 | 0.794 | 0.141 |
| 6 mon | 0.164 ± 0.038‡ | 0.178 ± 0.066 | 0.190 ± 0.052‡ | 0.073 | 0.651 | 0.073 | 0.758 |
| TISA at 750 μm (mm2) | |||||||
| Preoperation | 0.219 ± 0.077 | 0.334 ± 0.140 | 0.259 ± 0.107 | 0.002 | 0.002 | 0.214 | 0.048 |
| 6 mon | 0.315 ± 0.067‡ | 0.334 ± 0.132 | 0.357 ± 0.087‡ | 0.125 | 0.810 | 0.125 | 0.705 |
| TIA at 500 μm (°) | |||||||
| Preoperation | 33.4 ± 10.3 | 44.8 ± 12.4 | 36.9 ± 12.8 | 0.013 | 0.013 | 0.509 | 0.103 |
| 6 mon | 47.3 ± 7.7‡ | 46.3 ± 12.6 | 49.0 ± 7.9‡ | 0.460 | 0.986 | 0.550 | 0.631 |
| TIA at 750 μm (°) | |||||||
| Preoperation | 32.3 ± 10.25 | 42.8 ± 12.3 | 36.0 ± 12.7 | 0.017 | 0.018 | 0.383 | 0.177 |
| 6 mon | 46.2 ± 5.8† | 44.4 ± 12.1 | 47.9 ± 6.9† | 0.322 | 0.762 | 0.625 | 0.360 |
Table 3
Correlation analysis between ΔIOP and preoperative anterior-segment parameters
| Preoperative parameter | ΔIOP | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Cataract group | Vitrectomy group | Combined group | ||||
|
|
|
|
||||
| r | p-value* | r | p-value* | r | p-value* | |
| Angle opening distance (μm) | ||||||
| At 500 μm | 0.410 | 0.012 | - | - | 0.425 | 0.007 |
| At 750 μm | 0.442 | 0.006 | 0.326 | 0.256 | 0.427 | 0.007 |
| Trabecular-iris space area (μm) | ||||||
| At 500 μm | 0.170 | 0.315 | - | - | 0.438 | 0.005 |
| At 750 μm | 0.446 | 0.006 | 0.365 | 0.200 | 0.445 | 0.005 |
| Trabecular-iris angle (°) | ||||||
| At 500 μm | 0.342 | 0.038 | - | - | 0.340 | 0.034 |
| At 750 μm | 0.390 | 0.017 | 0.427 | 0.127 | 0.384 | 0.016 |
Table 4
Correlation analysis between the preoperative ACD, LT, and LV in the cataract and combined groups
| Variable | Preoperative LT | Preoperative LV | Preoperative ACD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Cataract group | Combined group | Cataract group | Combined group | Cataract group | Combined group | |||||||
|
|
|
|
|
|
|
|||||||
| r | p-value* | r | p-value* | r | p-value* | r | p-value* | r | p-value* | r | p-value* | |
| Preoperative LT | - | - | - | - | 0.673 | <0.001 | 0.713 | <0.001 | −0.708 | <0.001 | −0.808 | <0.001 |
| Preoperative LV | 0.673 | <0.001 | 0.713 | <0.001 | - | - | - | - | −0.808 | <0.001 | −0.799 | <0.001 |
| Preoperative ACD | −0.708 | <0.001 | −0.808 | <0.001 | −0.808 | <0.001 | −0.799 | <0.001 | - | - | - | - |



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print




