Relative Risks for Dementia among Individuals with Glaucoma: A Meta-Analysis of Observational Cohort Studies
Article information
Abstract
Purpose
To investigate the relative risks (RRs) for dementia among individuals with glaucoma.
Methods
We conducted a search of PubMed, Web of Science, Scopus, and Cochrane databases for observational cohort studies examining the association between glaucoma and dementia until March 2023. Two authors independently screened all titles and abstracts according to predefined inclusion and exclusion criteria. Pooled RR and 95% confidence intervals (CIs) were generated using random-effect models.
Results
The meta-analysis included 18 cohort studies conducted in eight countries and involving 4,975,325 individuals. The pooled RR for the association between glaucoma and all-cause dementia was 1.314 (95% CI, 1.099–1.572; I2 = 95%). The pooled RRs for the associations of open-angle glaucoma with Alzheimer dementia and Parkinson disease were 1.287 (95% CI, 1.007–1.646; I2 = 96%) and 1.233 (95% CI, 0.677–2.243; I2 = 73%), respectively. The pooled RRs for the associations of angle-closure glaucoma with all-cause dementia and Alzheimer dementia were 0.978 (95% CI, 0.750–1.277; I2 = 17%) and 0.838 (95% CI, 0.421–1.669; I2 = 16%), respectively. No evidence of publication bias was detected in the Begg-Mazumdar adjusted rank correlation test (p = 0.47).
Conclusions
Based on current observational cohort studies, there is evidence supporting that glaucoma is a risk factor for dementia in the adult population.
The brain and eye have similar embryonic origins, precise neuronal layers, complex neurochemistry, microglia, astroglia, blood barriers, and microvasculature [1]. The optic nerve’s axons form a direct connection between the retina and the brain, making the eyes an extension of the central nervous system that can provide valuable information on brain health [2,3].
Both glaucoma and dementia are neurodegenerative diseases. Glaucoma is characterized by chronic progressive optic neuropathy that leads to the degeneration of retinal ganglion cells (RGCs)—neurons that have axons extending into the brain [4,5]. RGC loss in glaucoma is thought to result from axonal transport discontinuity, similar to dementia, including Alzheimer disease (AD), which is also linked to defective axonal transport [6–8]. Common pathologic features of neurodegenerative conditions lead to cognitive and visual dysfunction in both glaucoma and dementia.
Previous epidemiological studies investigating the relationship between glaucoma and dementia have produced inconsistent results. Furthermore, meta-analysis studies on the connection between these two diseases have generated conflicting findings [9,10]. This study aims to establish the relative risks (RRs) for dementia among individuals with glaucoma.
Materials and Methods
Search strategy and selection criteria
This systematic review is registered in PROSPERO (Prospective Register of Systematic Reviews) on July 2022 (No. CRD42022340761). We conducted a systematic search of the PubMed, Embase, Scopus, and Cochrane Library databases to identify relevant studies. Our search strategies were developed in consultation with an academic librarian experienced in systematic review methods and utilized established MeSH (Medical Subject Headings) and Embase search terms where available. The search terms included glaucoma, open-angle glaucoma (OAG), normal-tension glaucoma (NTG), angle-closure glaucoma (ACG), dementia, AD, risk factor, determinant, and association. The details of our database search are provided in Supplementary Table 1. Two independent investigators (MGH and JL) conducted a masked literature search, with any discrepancies resolved through discussion and consensus or, if necessary, adjudicated by a third investigator (YKK). We also manually reviewed the reference lists of retrieved articles to identify additional relevant studies. Our search was limited to reports published through March 31, 2023. Full-text articles from eligible studies were included based on the following inclusion and exclusion criteria.
The inclusion criteria were the following: (1) observational cohort study; (2) glaucoma reported as covariate; (3) dementia as outcome measure; and (4) measure of association reported as RR, odds ratios, or relative risks with 95% confidence intervals (CIs), or the allowed calculation from the data presented in the article.
The exclusion criteria were the following: (1) not conducted with humans or adults; (2) narrative and/or systematic reviews, commentaries, and case reports; and (3) lacking diagnostic criteria for glaucoma and dementia. In cases where multiple publications reported on the same study population, we included only the study with the largest cohort after checking for duplicate analyses.
Data extraction and quality evaluation
Data extraction was performed by two investigators (MGH and JL) in an independent and masked manner using a standardized method based on the Cochrane Library’s Database of Systematic Reviews, as previously reported [11–15]. The extracted data were entered into a dedicated database and reviewed by a third investigator (YKK). The data extracted included the name of the first author, year of publication, study design characteristics, baseline study dates, country of study, number of participants, number of cases of dementia and glaucoma, population characteristics such as age and sex, follow-up durations, and adjusted confounding factors. To evaluate the quality of the studies, we used the Newcastle-Ottawa Scale [16] for assessment of comparative nonrandomized study quality and assessed the risk of selection, comparability, exposure and outcome, or any other forms of bias.
Statistical models for meta-analysis
The fully adjusted, study-specific RRs were combined to estimate the pooled RR with 95% CI based on a random effects model, as previously reported [11–15]. We quantified interstudy heterogeneity using the I2 statistic representing the percentage of interstudy variation attributable to heterogeneity (not to sampling error) [17,18]. Values of approximately 25%, 50%, and 75% represent low, medium, and high heterogeneity, respectively.
The researchers conducted predefined subgroup analyses to assess the impact of various study characteristics on the results. These included the types of glaucoma studied (OAG and ACG), as well as the subtypes of dementia (all-cause dementia, AD, and Parkinson disease).
We assessed publication bias using both qualitative and quantitative methods. The funnel plot was used as a visual tool to detect the potential absence of small studies with small effect sizes [19], while the Begg-Mazumdar adjusted rank correlation test [20] was used as a direct statistical analog to the funnel plot. The latter test determined whether a significant correlation existed between the effect estimates and their variances, indicating that studies had been selected in an unbiased way.
The data handling and statistical analyses were conducted by a statistician (YKK), who is an expert in meta-analysis. All of the 95% CIs and p-values were two-sided, with a p-value of less than 0.05 considered statistically significant. We used R ver. 4.3.4 (The R Foundation for Statistical Computing) for all statistical analyses.
Results
Study selection and appraisal
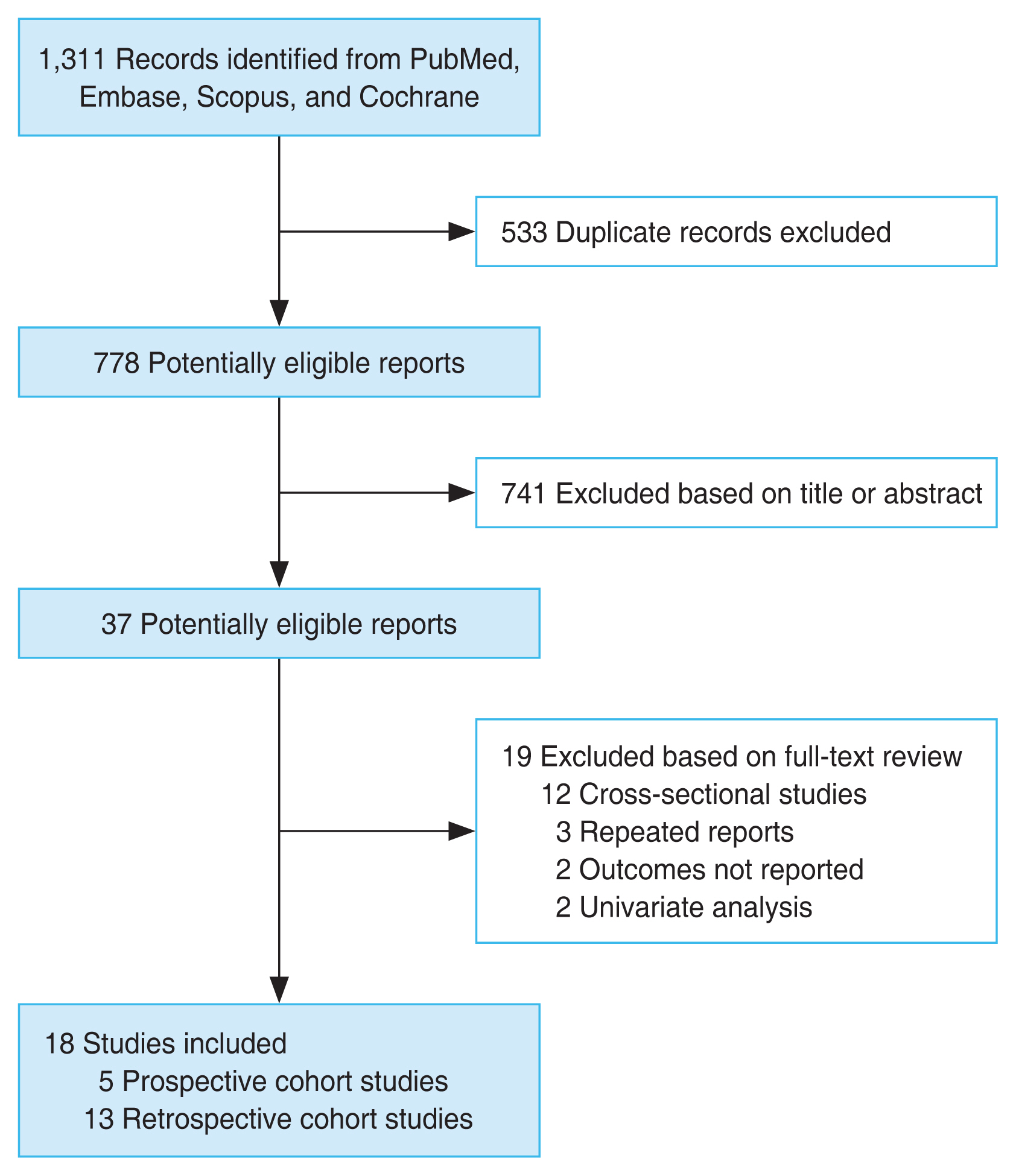
Fig. 1 summarizes the process of the database search. Initially, a total of 1,311 articles were found through the literature search of the PubMed, Embase, Scopus, and Cochrane Library databases. Of these 778 were left after removing 533 duplicates. Out of these, 741 articles were excluded after screening the titles and abstracts mainly because they were not relevant to the purpose of the meta-analysis. After a full-text review of the remaining 37 potentially relevant articles, 19 studies were excluded due to various reasons as listed in Fig. 1. In the end, a total of 18 observational studies [21–38], including 4,975,325 participants, were included for the meta-analysis.
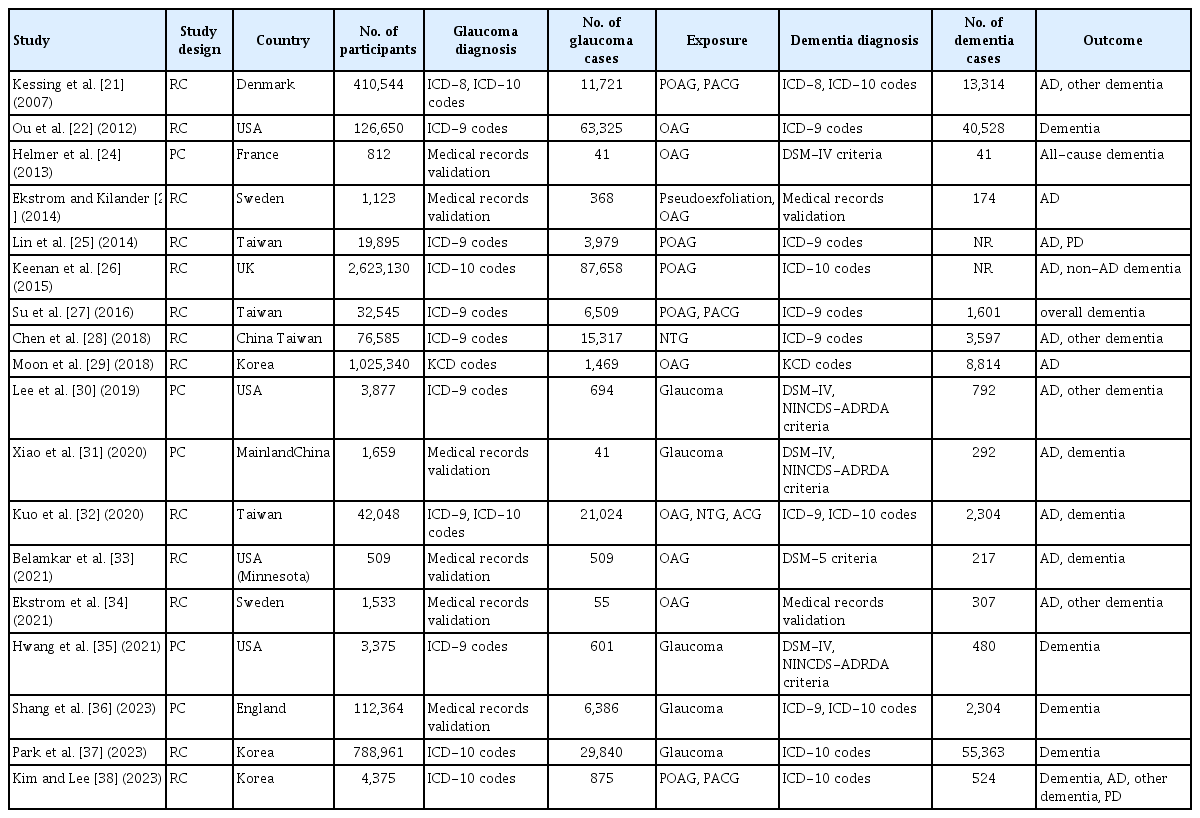
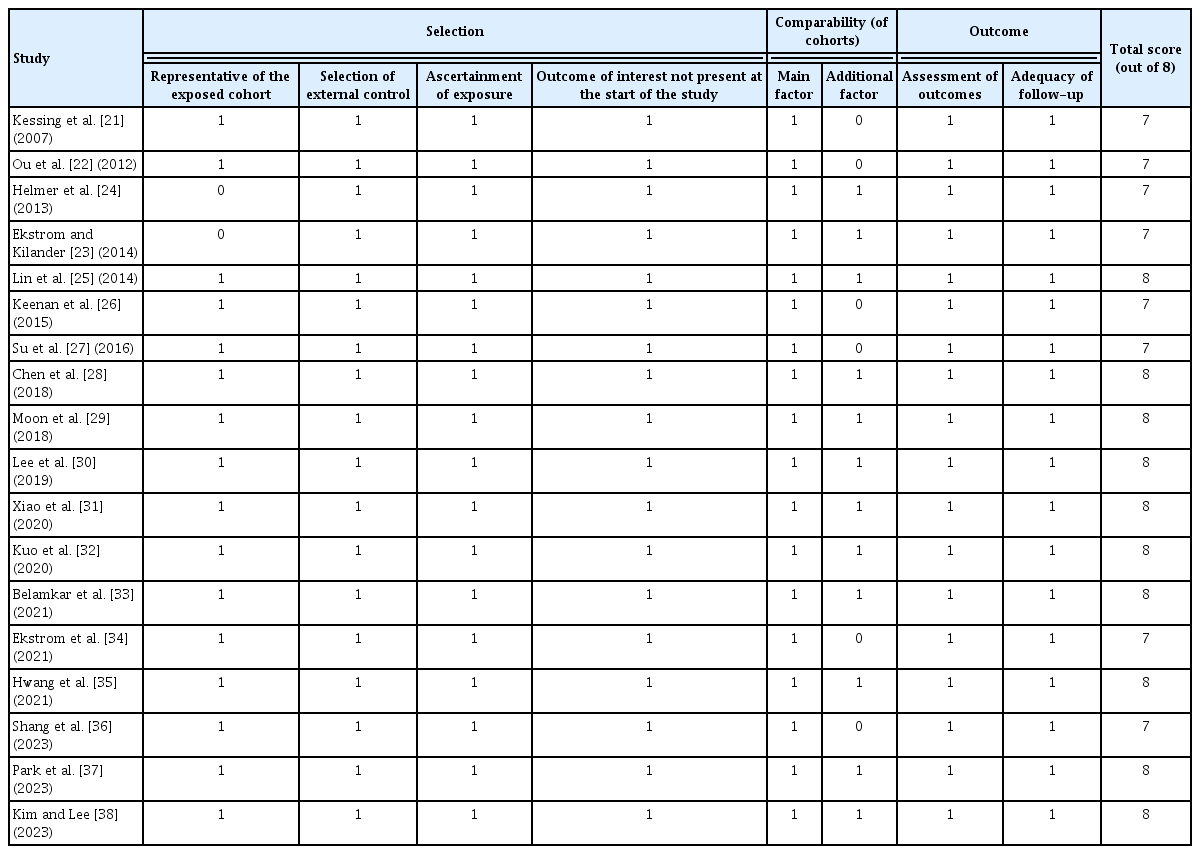
The characteristics of the included studies were summarized in Table 1 [21–38]. These studies were published between 2007 and 2023, and eight studies had been conducted in Asia, six in Europe, and four in the United States. Regarding study design, five of them were prospective cohort studies, while the remaining 13 were retrospective cohort studies. All of the studies included adult populations. Glaucoma was diagnosed with eye examination in two studies [24,34], with medical chart review in four studies [23,29,31,33], and with International Classification of Diseases (ICD) codes or national disease classification codes in 12 studies [21,22,25–28,30,32,35–38]. Outcomes of AD and dementia were diagnosed with the Diagnostic and Statistical Manual of Mental Disorders (DSM), Fourth or Fifth Edition or the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria in five studies [24,30,31,33,35], with medical chart evaluation in two studies [23,34], and with ICD or national disease classification codes in 11 studies [21,22,25–29,32,36–38]. The Newcastle-Ottawa Scale was applied to all of the included studies to assess the methodologic quality (Table 2) [21–38].
Association between glaucoma and dementia
Eighteen studies [21–38] reported the association between glaucoma and incidence of all-cause dementia in adult population (Fig. 2). Results of meta-analysis showed that adult patients with glaucoma was independently associated with an increased incidence of all-cause dementia (adjusted RR, 1.314; 95% CI, 1.099–1.572) with significant heterogeneity (p < 0.001, I2 = 95%).
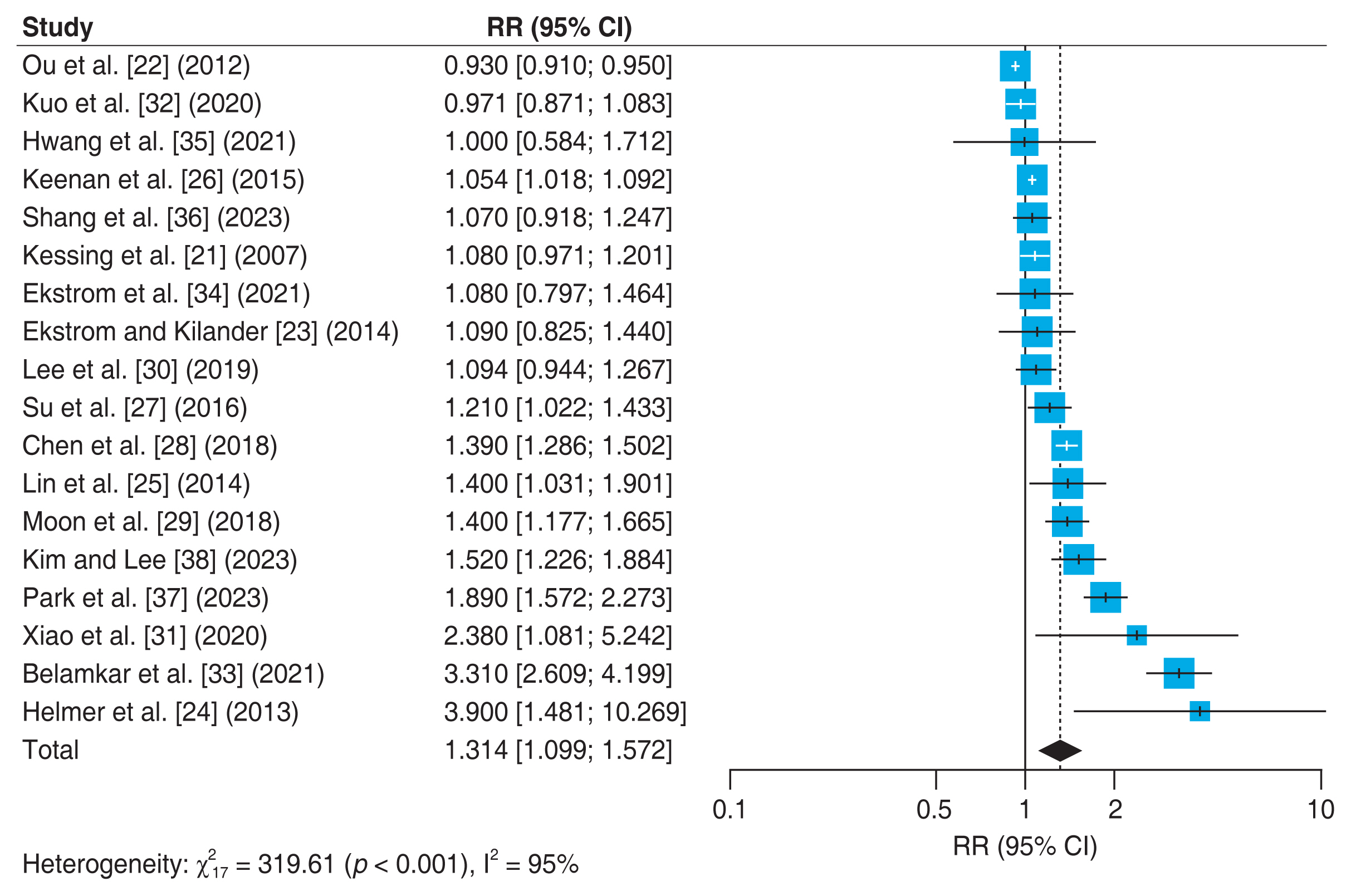
Forest plot of risk estimates of association between glaucoma and all-cause dementia. The size of the box representing the point estimate for each study in the forest plot in proportion to the contribution of that study’s weight estimate to the summary estimate. The horizontal lines indicate the 95% confidence intervals (CIs). The diamond denotes the pooled risk ratio, and the lateral tips of the diamond indicate the associated CIs. RR = relative risk.
Association between OAG and dementia
Fifteen studies [21–23,25,26,28–35,37,38] reported the association between OAG and incidence of AD (Fig. 3A). One of these studies by Chen et al. [28] analyzed the association between NTG and AD, and we conducted a meta-analysis including NTG in the OAG group. The meta-analysis showed that OAG was independently associated with an increased incidence of AD (adjusted RR, 1.287; 95% CI, 1.007–1.646; I2 = 96%; p < 0.001). Four studies [25,29,32,38] reported the association between OAG and Parkinson disease (Fig. 3B). The analysis about the association between OAG and Parkinson disease showed that OAG was not independently associated with an increased incidence of Parkinson disease (adjusted RR, 1.233; 95% CI, 0.677–2.243; I2 = 73%; p = 0.01).
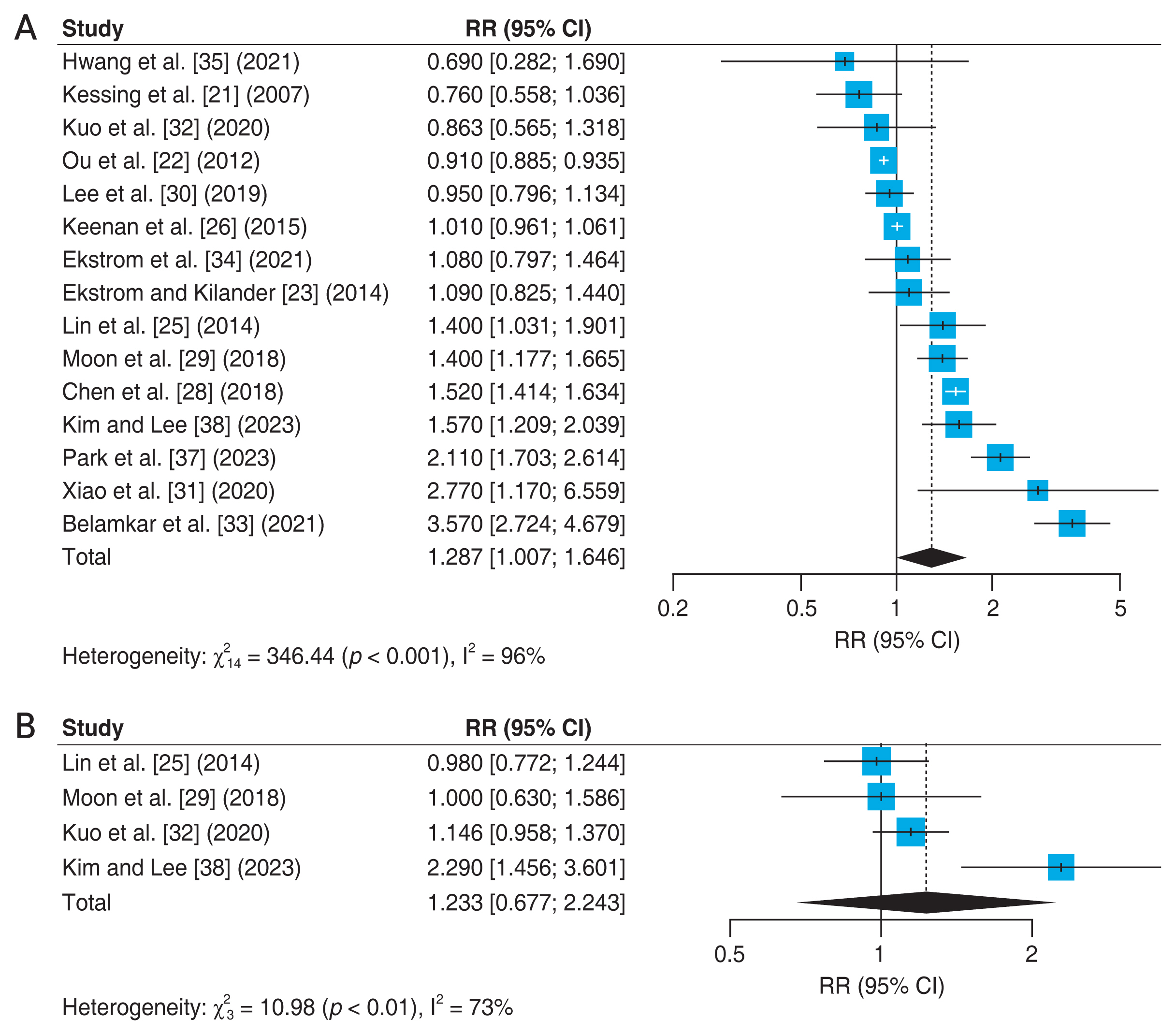
Forest plot of risk estimates. (A) Association between open-angle glaucoma and Alzheimer disease. (B) Association between open-angle glaucoma and Parkinson disease. The size of the box representing the point estimate for each study in the forest plot in proportion to the contribution of that study’s weight estimate to the summary estimate. The horizontal lines indicate the 95% confidence intervals (CIs). The diamond denotes the pooled risk ratio, and the lateral tips of the diamond indicate the associated CIs. RR = relative risk.
Association between ACG and dementia
Three studies [27,32,38] reported the association between ACG and incidence of all-cause dementia in adult population (Fig. 4A). Results of meta-analysis showed that adult patients with ACG was not independently associated with an increased incidence of all-cause dementia (adjusted RR, 0.978; 95% CI, 0.750–1.277) with low heterogeneity (p = 0.30, I2 = 17%). Three studies [21,32,38] reported the association between ACG and AD (Fig. 4B). The analysis about the association between ACG and AD also showed that ACG was not independently associated with an increased incidence of AD (adjusted RR, 0.838; 95% CI, 0.421–1.669; I2 = 16%; p = 0.30).

Forest plot of risk estimates. (A) Association between angle-closure glaucoma and all-cause dementia. (B) Association between angle-closure glaucoma and Alzheimer disease. The size of the box representing the point estimate for each study in the forest plot in proportion to the contribution of that study’s weight estimate to the summary estimate. The horizontal lines indicate the 95% confidence intervals (CIs). The diamond denotes the pooled risk ratio, and the lateral tips of the diamond indicate the associated CIs. RR = relative risk.
Publication bias
Fig. 5 is a funnel plot depicting publication bias. The MG Huh, et al. Risks for Dementia in Patients with Glaucoma funnel plot shows a relatively symmetrical patterns, but the small study with a small effect size appears to be missing from the funnel plot in the bottom left. However, it has been verified that there is no publication bias through the Begg-Mazumdar adjusted rank correlation test (p = 0.47).
Discussion
This study found that glaucoma patients are at a greater risk of developing all-cause dementia than those without glaucoma, with OAG patients showing a higher incidence of AD. Several studies suggest commonalities in the pathophysiology supporting the association between glaucoma and AD. Glaucoma is characterized by RGC death and retinal nerve fiber layer thinning. Similarly, RGC loss was found in Alzheimer retinal tissue [39,40]. Amyloid-β, a neurotoxin, can induce RGC degeneration by disrupting synaptic circuitry and retrograde transport of neurotrophic factors in optic nerve axons [41–44]. The cerebrospinal fluid (CSF) circulatory failure may also cause oxidative stress and degeneration of RGCs in NTG [45,46]. Several studies suggest that low CSF pressure may contribute to the pathogenesis of both diseases, but low intracranial pressure alone cannot fully explain the association [47–50]. The glymphatic system, a recently defined paravascular channel system, may also contribute to the lack of amyloid beta clearance in both diseases [51–54]. On the other hand, the ACG group, a subgroup of glaucoma, was found to be unrelated to the development of dementia or AD. ACG has several risk factors that are different from OAG, such as hyperopic eyes, female sex, shallow anterior chamber, and short axial length [55,56]. In addition, in the pathophysiology of ACG, unlike OAG, the anterior chamber angle is structurally blocked, which impairs aqueous humor outflow, which increases intraocular pressure. Sometimes, it causes a sudden and high increased intraocular pressure, leading to the rapid progression of glaucoma [55–58]. On the other hand, OAG often shows a gradual progression of glaucoma due to relatively long-term ocular hypertension, and in a broader sense, NTG may also fall under the spectrum of OAG, which means that even in the normal range of intraocular pressure, the optic nerve and optic nerve fibers undergo glaucoma progression [59,60]. Therefore, differences in the course of glaucoma progression between ACG and OAG may exist. The angle structure plays a more important role in the progression of glaucoma in ACG, there may be a lack of correlation with dementia.
Previous meta-analysis studies of the association between glaucoma and dementia
Xu et al. [9] conducted the earliest meta-analysis (up to January 2018) on the association between glaucoma and dementia, which included eight observational studies. Their findings showed that individuals with glaucoma had a higher risk of AD (RR, 1.52; 95% CI, 1.41–1.63; I2 = 97%; p < 0.001), specifically primary OAG (RR, 1.52; 95% CI, 1.41–1.63; I2 = 97%; p < 0.001). Zhao et al. [10] analyzed 11 cohort studies, including 4,645,925 participants, and found no independent association between glaucoma and all-cause dementia, AD, or non-AD. The most recent meta-analysis by Xu et al. [61] examined the prevalence of ocular diseases and cognitive impairment or dementia, reporting a range of 2.4% to 3.3% for dementia prevalence and 3.3% to 78.6% for cognitive impairment prevalence among people with glaucoma. Cumulative incidence of dementia ranged from 2.2% to 12.4%. The aforementioned meta-analysis performed an investigation into the relationship between glaucoma and dementia, including 8 to 11 studies published up until 2020. This study included a total of 18 cohorts, including the most recent research published from 2020 onwards. Accordingly, this study appropriately included a diverse selection of studies from Asia, Europe, and the Americas, maintaining objectivity without favoring any particular region. Furthermore, the study exclusively selected for cohort studies to assess whether there was significant difference in dementia incidence between groups exposed to glaucoma and those not exposed, as well as to explore potential associations. Upon reviewing the results of this study, the relationship between glaucoma and dementia, OAG and AD showed results corresponding to the two studies mentioned above [9,61], and although this result was opposite to the study by Zhao et al. [10] which analyzed 11 cohort studies, it is our belief that the inclusion of a more extensive array of studies and a larger participant pool in this current study lends additional weight and credibility to our analysis results.
Limitations
The study has limitations that need to be noted. Firstly, the diagnoses of dementia and glaucoma relied on claims data, making it difficult to validate accuracy using medical records. Dementia diagnosis typically requires specialized tests, but in this study, it relied on ICD codes, medical records, or neurological examinations supplemented by the Mini-Mental State Examination (MMSE). The study included participants from National Health Insurance Service (Wonju, Korea), which provided data from a large population cohort. However, relying on ICD codes limited the classification of different dementia subtypes with varying causes. Despite this limitation, certain studies separately analyzed AD, allowing for a meta-analysis on the relationship between glaucoma and AD. Secondly, we could not analyze the prevalence of dementia based on the severity of glaucoma, as moderate to severe glaucoma causes visual impairment leading to dementia through sensory deprivation. Furthermore, research on the prevalence of other glaucoma subtypes and dementia is needed. Lastly, population-based studies, which this study is based on, require health insurance or multi-center data, making it a limitation only possible in developed countries. This study’s representativeness to the global population may be questioned. Nonetheless, it’s significant as it performed a meta-analysis by gathering as many population-based studies as possible.
Conclusions
This study found that glaucoma patients may have a higher risk of dementia compared to those without the condition. Additionally, OAG has been linked to AD prevalence, but further research is needed to fully understand the mechanisms behind this relationship. The results corroborate earlier findings, enhancing our understanding of the connection between glaucoma and dementia, especially AD. Despite some limitations, such as reliance on claims data for diagnosis, this study highlights the importance of investigating the relationship between glaucoma and dementia. Future studies should investigate these associations in more detail and investigate the underlying mechanisms to develop targeted interventions for prevention and management. In summary, this meta-analysis provides valuable insights into the complex relationship between glaucoma and dementia, advancing our knowledge of these neurodegenerative diseases and their potential shared causes.
Acknowledgements
None.
Notes
Conflicts of Interest
None.
Funding
This study was supported by a National Research Foundation of Korea (NRF) grant (No. NRF-2022R1F1A1064186). The funder had no role in the initiation or design of the study, collection of samples, analysis, interpretation of data, paper writing or submission for publication. The study and researchers are independent of the funder.
Supplementary Materials
Supplementary Table 1.
Search strategy
Supplementary materials are available from https://doi.org/10.3341/kjo.2023.0059.