|
 |
| Korean J Ophthalmol > Volume 37(6); 2023 > Article |
|
Abstract
Purpose
This study aimed to investigate changes in cytokine levels after intravitreal bevacizumab injection in patients with chronic central serous chorioretinopathy (CSC).
Methods
In a prospective interventional trial, 12 eyes from 12 patients with chronic CSC and six eyes from six patients who underwent cataract surgery were included as controls. Patients diagnosed as with CSC received a single intravitreal injection of bevacizumab (1.25 mg/0.05 mL). Aqueous humor samples were collected from the patients and controls. Best-corrected visual acuity and foveal thickness were evaluated, and aqueous samples were obtained before and 4 weeks after injection. The aqueous concentrations of interleukin (IL)-6, IL-8, interferon-induced protein (IP)-10, monocyte chemotactic protein (MCP)-1, platelet-derived growth factor (PDGF)-AA, and vascular endothelial growth factor (VEGF) were measured using a multiplex bead assay.
Results
After injection, the foveal thickness decreased significantly from 328.08 μm (range, 210-477 μm) to 283.91 μm (range, 168-356 μm; p = 0.048), but the best-corrected visual acuity was not significantly different (p = 0.066). The aqueous levels of IL-8 increased significantly from 3.3 pg/mL (range, 1.5-8.3 pg/mL) to 4.7 pg/mL (range, 2.2-11.6 pg/mL) at 4 weeks after the injection (p = 0.046). The aqueous levels of VEGF decreased significantly from 31.4 pg/mL (range, 17.0-53.3 pg/mL) to 15.2 pg/mL (range, 7.7-21.5 pg/mL; p < 0.01). No significant changes in levels of IL-6 (p = 0.455), IP-10 (p = 0.055), MCP-1 (p = 0.076), and PDGF-AA (p = 0.339) were noted 4 weeks after injection.
Conclusions
In this study we found intravitreal bevacizumab injection decreased VEGF and increased IL-8 in the eyes of patients with chronic CSC. This study suggests the possibility that the pathogenesis of CSC may be related to abnormal circulation of the choroidal blood vessels through VEGF and IL-8 cytokine level changes.
Central serous chorioretinopathy (CSC) is a chorioretinal disease characterized by the development of serous retinal detachment at the posterior pole, which can cause visual impairment [1,2]. In most eyes of patients with CSC, serous retinal detachment resolves without treatment; however, some patients develop visual loss from persistent serous retinal detachment, cystoid macular degeneration, or retinal pigment epithelium decompensation [2,3].
As chronic CSC has been reported to lead to visual dysfunction, further treatment for CSC is warranted. Traditionally, laser photocoagulation and photodynamic therapy with verteporfin have been shown to be beneficial in the treatment for chronic CSC [4-6]. Photodynamic therapy with verteporfin has become a popular treatment option for chronic and recurrent cases because it reduces choroidal congestion and vascular hyperpermeability. Recently, anti-vascular endothelial growth factor (anti-VEGF) therapy has been widely used to treat chronic CSC. Intravitreal bevacizumab injection (IVBe) for the treatment of CSC has been shown in several studies to be safe and effective with functional and anatomical benefits [7-10]. However, no studies have measured the levels of multiple intraocular cytokines in the eyes of patients with chronic CSC.
To the best of our knowledge, there have been no evaluations of the effects of intravitreal bevacizumab injections on the aqueous humor cytokine levels in chronic CSC. Therefore, this study examined the changes in aqueous inflammatory and angiogenic cytokine levels after intravitreal bevacizumab injection to reduce subretinal fluid (SRF) in chronic CSC.
This study was approved by the Institutional Review Board of Gachon University (No. GCIRB2015-15). The study followed the tenets of the Declaration of Helsinki. All study participants provided informed consent after understanding the nature and the possible consequences of the study.
This study was a prospective interventional case series. All the investigations were conducted in accordance with the principles of the Declaration of Helsinki. This prospective study was conducted with the informed consent of the patients and with a small number of patients. We conducted this study as a pilot study.
CSC was defined as detachment of the neurosensory retina from the macula caused by idiopathic or diffuse leakage from the retinal pigment epithelium (RPE). Leakage from the RPE was detected using fluorescein angiography (FA), and choroidal vascular hyperpermeability was detected using indocyanine green angiography. Inclusion criteria of chronic CSC were as follows: (1) symptoms like blurred vision or metamorphopsia more than 3 months; (2) persistent or recurrent SRF on optical coherence tomography (OCT); (3) diffuse abnormality of the outer retinal layer or RPE on OCT; and (4) various leakage patterns of CSC on FA (Table 1).
Twelve patients with chronic CSC were recruited at the Department of Ophthalmology, Gachon University Gil Medical Center (Incheon, Korea) between October 2016 and April 2018. Patients with chronic CSC received intravitreal injection of 1.25 mg of bevacizumab. The control group comprised six patients who had undergone cataract surgery without other ocular or systemic diseases. Prior to and after treatment, all patients underwent a comprehensive ophthalmic examination that included the best-corrected visual acuity (BCVA) according to the Snellen chart, intraocular pressure (IOP) measurement, slit-lamp examination, fundus examination, and central foveal thickness (CFT) measurement using Stratus OCT 3 (Carl Zeiss Meditec Inc) using a fast macular scan protocol.
Intravitreal injections of 1.25 mg/0.05 mL bevacizumab (Avastin, Genentech Inc) was done. All the injections were performed by a retinal specialist (DYL) using topical anesthesia. Under sterile conditions in the operating room, bevacizumab was injected via a 30-gauge needle through the pars plana 3.5 mm posterior to the limbus. Two weeks after the injection, levofloxacin (Cravit, Santen) ophthalmic solution was administered four times a day.
Undiluted aqueous humor samples (50-100 μL) were obtained immediately before an intravitreal injection of bevacizumab, 4 weeks after the injection in the CSC group, and at the beginning of cataract surgery in the control group. All sample collections were performed by a single physician (DYL) under sterile conditions in the operating room. The aqueous humor was withdrawn through a limbal paracentesis site using a 30-gauge needle with a tuberculin syringe. Special care was taken to avoid touching the intraocular tissues and prevent mixing of aqueous samples with other fluids. The specimens were transferred immediately to sterile plastic tubes and stored at −70 °C until assayed. Interleukin (IL)-6, IL-8, interferon-induced protein (IP)-10, monocyte chemotactic protein (MCP)-1, platelet-derived growth factor (PDGF)-AA, and VEGF levels in the aqueous samples were measured using the Luminex 100 Multiplex Array Assay (Luminex Corp). The median data using a five-parameter logistic or spline curve-fitting method were saved and evaluated to calculate the cytokine/chemokine concentrations in the samples.
As this study has invasive procedure, sampling aqueous by anterior chamber paracentesis, we had patients’ consent before procedure and explained in detail about the procedure, and possible complications. We checked patients’ visual acuity, intraocular pressure, anterior chamber change, fundus examination the next day and 3 days after anterior chamber paracentesis to check out the possible complications.
All statistical analyses were performed using the SPSS ver. 12.0 (SPSS Inc). The aqueous levels of IL-6, IL-8, IP-10, MCP-1, PDGF-AA, and VEGF are expressed as mean ± standard deviation. To analyze the statistical differences, the Wilcoxon signed rank test was used between the preinjection and postinjection clinical data, and a Mann-Whitney U-test was used to compare the control with the CSC groups. Statistical significance was set at p < 0.05.
Twelve eyes from 12 patients with CSC and six eyes from six patients who underwent cataract surgery (control) were included in this study. The mean age was 48.7 ± 9.2 years in the patients with CSC and 51.8 ± 5.7 years in the control group (p = 0.148). One of the 12 patients in the CSC group had a history of hypertension. In the control group, one of six patients had a history of hypertension. The mean SRF was 7.7 ± 2.5 months (Table 2).
In the CSC group, BCVA (logarithm of the minimum angle of resolution, logMAR) slightly improved from 0.43 logMAR (range, 0.15-1.0 logMAR) to 0.27 logMAR (range, 0.15-0.30 logMAR) at 4 weeks after the injection but there were no significant differences (p = 0.066). No significant changes in IOP were observed (p = 0.534). The CFT (μm) in the CSC group decreased significantly from 328.08 μm (range, 210-477 μm) to 283.91 μm (range, 168-356 μm) 4 weeks after the injection (p = 0.048) (Table 3 and Fig. 1A, 1B). The levels of cytokines in the aqueous humor are listed in Table 4. In the measurements of IL-6, IL-8, IP-10, MCP-1, PDGF-AA, and VEGF, no samples were below the detection limit in patients and controls. Cytokine levels were not significantly different between the CSC and control groups (p = 0.316, p = 0.517, p = 0.303, p = 0.558, p = 0.153, and p = 0.631, respectively).
In the CSC group, the aqueous levels of IL-8 increased significantly from 3.3 pg/mL (range, 1.5-8.3 pg/mL) to 4.7 pg/mL (range, 2.2-11.6 pg/mL) at 4 weeks after the injection (p = 0.046). The aqueous levels of VEGF decreased significantly from 31.4 pg/mL (range, 17.0-53.3 pg/mL) to 15.2 pg/mL (range, 7.7-21.5 pg/mL) 4 weeks after the injection (p < 0.010) (Fig. 2A-2F). No significant changes in levels of IL-6 (p = 0.455), IP-10 (p = 0.055), MCP-1 (p = 0.076), and PDGF-AA (p = 0.339) were noted 4 weeks after injection (Table 5).
No severe adverse events (retinal detachment, endophthalmitis, or vitreous hemorrhage) or systemic side effects such as elevated blood pressure or thromboembolic events were observed throughout the study.
CSC is a common macular disease that often presents with well-circumscribed serous retinal detachment in the macular region on clinical examination, with one or several leakage points at the level of the RPE on FA [11]. Acute CSC is typically a self-limiting process with few recognized visual sequelae, while chronic CSC and recurrent CSC may lead to RPE atrophy and neurosensory retinal changes that result in permanent loss of visual function [12]. Recently, Mrejen et al. [13] reported that 12.8% of 133 patients with chronic CSC progressed to bilateral legal blindness.
Focal laser and photodynamic therapy have proven effective for the treatment of CSC; however, focal laser is associated with permanent scotoma, which enlarges over time with RPE scar expansion, as well as possible development of choroidal neovascularization [4]. Photodynamic therapy is expensive, and cases of secondary RPE change and choriovascular neovascularization after treatment for CSC have been reported [14-16].
Recently, there have been several reports that IVBe results in vision improvement and reduced neurosensory detachment in patients with acute and chronic CSC [7-9]. Based on the literature, one study [17] demonstrated that the VEGF level in aqueous humor of patients with CSC is higher than that of the normal population; however, another study [18] showed that VEGF levels did not differ significantly.
The reasons for the differences in the results between the studies are as follows. First, unlike diabetic retinopathy caused by extensive retinal ischemia, CSC is caused by local ischemia of the choroid; therefore, the range of increase in VEGF concentration is not large. Second, when the VEGF concentration was measured from the anterior chamber, a smaller amount than the choroid or vitreous concentration was reflected. Finally, it may be difficult to show statistical differences with a small sample size because the degree of change in the VEGF concentration for each individual is large. To compensate for these points, this study aimed to investigate cytokine changes before and after anti-VEGF treatment in patients with chronic CSC.
VEGF is a potent angiogenic factor that plays a major role in increasing vascular permeability in retinal vascular disorders. Choroidal ischemia may induce an increase in the concentration of VEGF, which may contribute to the pathogenesis of CSC. Therefore, intravitreal anti-VEGF therapy has been studied by various research groups [10,19-23]. However, there have been no studies regarding cytokines or growth factors after intravitreal anti-VEGF injections in chronic CSC. This study aimed to evaluate changes in aqueous inflammatory cytokine levels after IVBe in chronic CSC to better understand its pathogenesis.
In this study, no significant difference in the aqueous levels of IL-6, IP-10, MCP-1, PDGF-AA, and VEGF was observed between the chronic CSC and control groups before the injection. This could be due to the small sample size and aqueous VEGF levels, which did not completely reflect intraocular VEGF levels. Further investigation is needed to evaluate VEGF expression in vitreous and retinal lesions.
IL-8 is a proinflammatory and angiogenic cytokine known as a neutrophil chemotactic factor and T-cell activator in the innate immune system. IL-8 is produced by endothelial and glial cells in ischemic retina [24]. Increased aqueous humor levels of IL-8 have been reported in retinal artery occlusion, uveitis, and proliferative diabetic retinopathy [25-27]. Chronic inflammatory conditions often result from aberrant production of proinflammatory factors, such as chemokines. A chemokine that is implicated in chronic inflammation is IL-8 [28]. Lim et al. [18] showed that the aqueous humor levels of VEGF and IL-8 were not significantly increased in patients with CSC compared to those in the healthy control group. Deng et al. [29] reported that IL-8 levels were elevated and functioned as compensatory angiogenesis signaling in suppressing VEGF-A expression, which means that an increase in IL-8 may be associated with changes in VEGF levels.
In the present study, there were no significant differences in the aqueous humor level of IL-8 between the CSC and control groups. However, aqueous levels of IL-8 increased significantly after injection in chronic CSC patients (p = 0.046) (Table 5). Regardless of the therapeutic effects, IL-8 was the only cytokine showing an opposite response to the anti-VEGF injection in this study. We hypothesized that IVBe decreases VEGF levels, which could influence the compensatory increase in IL-8 levels. This result suggests that other upstream or parallel inflammatory pathways along with the VEGF signaling pathway may aggravate retinal ischemia, which is related to the pathogenesis of chronic CSC. Nevertheless, further investigation is required to elucidate the role of IL-8 in chronic CSC.
IL-6 is a multifunctional cytokine that is essential for the regulation of immune processes and induction of acute-phase reactions. It increases vascular permeability and angiogenesis by inducing VEGF [30]. IL-6 is produced by various cells, including fibroblasts, monocytes, T or B lymphocytes, vascular endothelial cells, and glial cells. Terao et al. [31] reported that aqueous IL-6 levels were significantly upregulated in the chronic CSC group compared to the acute CSC group. In this study, there were no differences in the changes in IL-6 levels after IVBe administration. This may be due to the relatively small sample size. One possible hypothesis is that IL-6 has little influence on chronic CSC, which is a chronic inflammatory state. IP-10 is induced in various cells in response to interferon-γ and lipopolysaccharide.
IP-10 promotes chemoattraction for monocytes and T lymphocytes but lacks the neutrophil chemoattractant and angiogenic properties of IL-8 [32]. MCP-1 is produced by retinal endothelial cells and has been implicated in leukostasis in hypoxic retinas [33,34]. It facilitates angiogenesis. PDGF is one of the most ubiquitous growth factors that stimulate cellular proliferation and direct cellular movement. This study demonstrated that IL-6, IP-10, MCP-1, and PDGF showed no significant changes in concentration after IVBe in chronic CSC. This suggests a less significant role for these factors in anti-VEGF therapy for chronic CSC.
The exact mechanisms underlying CSC pathogenesis and the beneficial effects of anti-VEGF treatment remain unclear. However, in our study, we documented the aqueous humor of patients with chronic CSC and the difference in cytokine levels after anti-VEGF treatment in chronic CSC. Our results suggest that other inflammatory upstream or parallel pathways along with the VEGF signaling pathway may play a role in the development of chronic CSC.
Our study had some limitations. A small sample size might limit the statistical power in detecting differences in cytokines or factors that would influence the results. Although the levels of cytokines in the aqueous humor may reflect those in the vitreous fluid, analysis of vitreous fluid would reflect intraocular cytokines more accurately. Additionally, cytokine levels may vary depending on the time of sample collection. However, to the best of our knowledge, this is the first study to document cytokine changes after anti-VEGF therapy in patients with chronic CSC. Our research has limitations in quantitatively analyzing choroidal ischemia. As this study did not perform ICG, so choroidal ischemia could not be analyzed quantitatively. Based on the pathology of chronic CSC disease, it is difficult to reflect the condition of focal choroidal ischemia by analyzing anterior chamber VEGF concentration. The strength of our research lies in the analysis of cytokine differences before and after anti-VEGF injection in chronic CSC patients. Our study reached the same conclusion as the experimental study by Zhou et al. [35] through cytokine analysis before and after anti-VEGF treatment, suggesting that it is related to choroidal ischemia.
According to a previous study by Zhou et al. [35], blockade of VEGF signaling augments IL-8 secretion via MEKERK1/2 axis and overactivation of VEGF pathway decreases IL-8 production in human RPE cells. Our study confirmed the difference in cytokine before and after injection and hypothesized that VEGF concentration was increased and IL-8 was decreased in chronic CSC with focal choroidal ischemia, but after anti-VEGF injection, VEGF concentration decreased and IL-8 was increased. This suggests the possibility of focal choroidal ischemia.
In conclusion, intravitreal bevacizumab injection reduces VEGF and increases IL-8 levels in multiple cytokine profiles in the eyes of patients with chronic CSC. This study is the first report comparing changes in cytokine levels in the anterior chamber before and after bevacizumab injection in CSC patients. Although the exact etiology of chronic CSC patients is unknown, this pilot study suggests the possibility that the pathogenesis of CSC may be related to abnormal circulation of the choroidal blood vessels through IL-8 cytokine level changes. Based on this study, a study based on a larger patient group will be conducted.
References
1. Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol 1967;63(Suppl):1-139.
2. Jung SH, Kim KA, Sohn SW, Yang SJ. Cytokine levels of the aqueous humour in central serous chorioretinopathy. Clin Exp Optom 2014;97:264-9.


3. Loo RH, Scott IU, Flynn HW Jr, et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina 2002;22:19-24.


4. Burumcek E, Mudun A, Karacorlu S, Arslan MO. Laser photocoagulation for persistent central serous retinopathy: results of long-term follow-up. Ophthalmology 1997;104:616-22.


5. Taban M, Boyer DS, Thomas EL, Taban M. Chronic central serous chorioretinopathy: photodynamic therapy. Am J Ophthalmol 2004;137:1073-80.


6. Chan WM, Lai TY, Lai RY, et al. Safety enhanced photodynamic therapy for chronic central serous chorioretinopathy: one-year results of a prospective study. Retina 2008;28:85-93.

7. Torres-Soriano ME, Garcia-Aguirre G, Kon-Jara V, et al. A pilot study of intravitreal bevacizumab for the treatment of central serous chorioretinopathy (case reports). Graefes Arch Clin Exp Ophthalmol 2008;246:1235-9.



8. Schaal KB, Hoeh AE, Scheuerle A, et al. Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eur J Ophthalmol 2009;19:613-7.



9. Seong HK, Bae JH, Kim ES, et al. Intravitreal bevacizumab to treat acute central serous chorioretinopathy: short-term effect. Ophthalmologica 2009;223:343-7.



10. Lim SJ, Roh MI, Kwon OW. Intravitreal bevacizumab injection for central serous chorioretinopathy. Retina 2010;30:100-6.


11. Yu J, Xu G, Chang Q, et al. Risk factors for persistent or recurrent central serous chorioretinopathy. J Ophthalmol 2019;2019:5970659.




12. Nicholson B, Noble J, Forooghian F, Meyerle C. Central serous chorioretinopathy: update on pathophysiology and treatment. Surv Ophthalmol 2013;58:103-26.



13. Mrejen S, Balaratnasingam C, Kaden TR, et al. Long-term visual outcomes and causes of vision loss in chronic central serous chorioretinopathy. Ophthalmology 2019;126:576-88.


14. Cardillo Piccolino F, Eandi CM, Ventre L, et al. Photodynamic therapy for chronic central serous chorioretinopathy. Retina 2003;23:752-63.


15. Chan WM, Lam DS, Lai TY, et al. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol 2003;87:1453-8.



16. Colucciello M. Choroidal neovascularization complicating photodynamic therapy for central serous retinopathy. Retina 2006;26:239-42.


17. Lim JW, Kim MU. The efficacy of intravitreal bevacizumab for idiopathic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol 2011;249:969-74.



18. Lim JW, Kim MU, Shin MC. Aqueous humor and plasma levels of vascular endothelial growth factor and interleukin-8 in patients with central serous chorioretinopathy. Retina 2010;30:1465-71.


19. Mehany SA, Shawkat AM, Sayed MF, Mourad KM. Role of Avastin in management of central serous chorioretinopathy. Saudi J Ophthalmol 2010;24:69-75.



20. Entezari M, Ramezani A, Yaseri M. Intravitreal bevacizumab for treatment of refractory central serous choroidoretinopathy. Korean J Ophthalmol 2012;26:139-42.



21. Iacono P, Battaglia Parodi M, et al. Central serous chorioretinopathy treatments: a mini review. Ophthalmic Res 2015;55:76-83.



22. Chung YR, Seo EJ, Lew HM, Lee KH. Lack of positive effect of intravitreal bevacizumab in central serous chorioretinopathy: meta-analysis and review. Eye (Lond) 2013;27:1339-46.




23. Park SU, Lee SJ, Kim M. Intravitreal anti-vascular endothelial growth factor versus observation in acute central serous chorioretinopathy: one-year results. Korean J Ophthalmol 2014;28:306-13.



24. Lee YS, Choi I, Ning Y, et al. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br J Cancer 2012;106:1833-41.




25. Kramer M, Goldenberg-Cohen N, Axer-Siegel R, et al. Inflammatory reaction in acute retinal artery occlusion: cytokine levels in aqueous humor and serum. Ocul Immunol Inflamm 2005;13:305-10.


26. Yuuki T, Kanda T, Kimura Y, et al. Inflammatory cytokines in vitreous fluid and serum of patients with diabetic vitreoretinopathy. J Diabetes Complications 2001;15:257-9.


27. Paroli MP, Teodori C, D’Alessandro M, et al. Increased vascular endothelial growth factor levels in aqueous humor and serum of patients with quiescent uveitis. Eur J Ophthalmol 2007;17:938-42.



28. Harada A, Mukaida N, Matsushima K. Interleukin 8 as a novel target for intervention therapy in acute inflammatory diseases. Mol Med Today 1996;2:482-9.


29. Deng Z, Zhou J, Han X, Li X. TCEB2 confers resistance to VEGF-targeted therapy in ovarian cancer. Oncol Rep 2016;35:359-65.


30. Funatsu H, Yamashita H, Ikeda T, et al. Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology 2003;110:1690-6.


31. Terao N, Koizumi H, Kojima K, et al. Association of upregulated angiogenic cytokines with choroidal abnormalities in chronic central serous chorioretinopathy. Invest Ophthalmol Vis Sci 2018;59:5924-31.


32. Ide N, Hirase T, Nishimoto-Hazuku A, et al. Angiotensin II increases expression of IP-10 and the renin-angiotensin system in endothelial cells. Hypertens Res 2008;31:1257-67.


33. Meleth AD, Agron E, Chan CC, et al. Serum inflammatory markers in diabetic retinopathy. Invest Ophthalmol Vis Sci 2005;46:4295-301.


Fig. 1
Scatter plot of (A) best-corrected visual acuity (BCVA) and (B) central foveal thickness (CFT) before and after injection. logMAR = logarithm of the minimum angle of resolution.
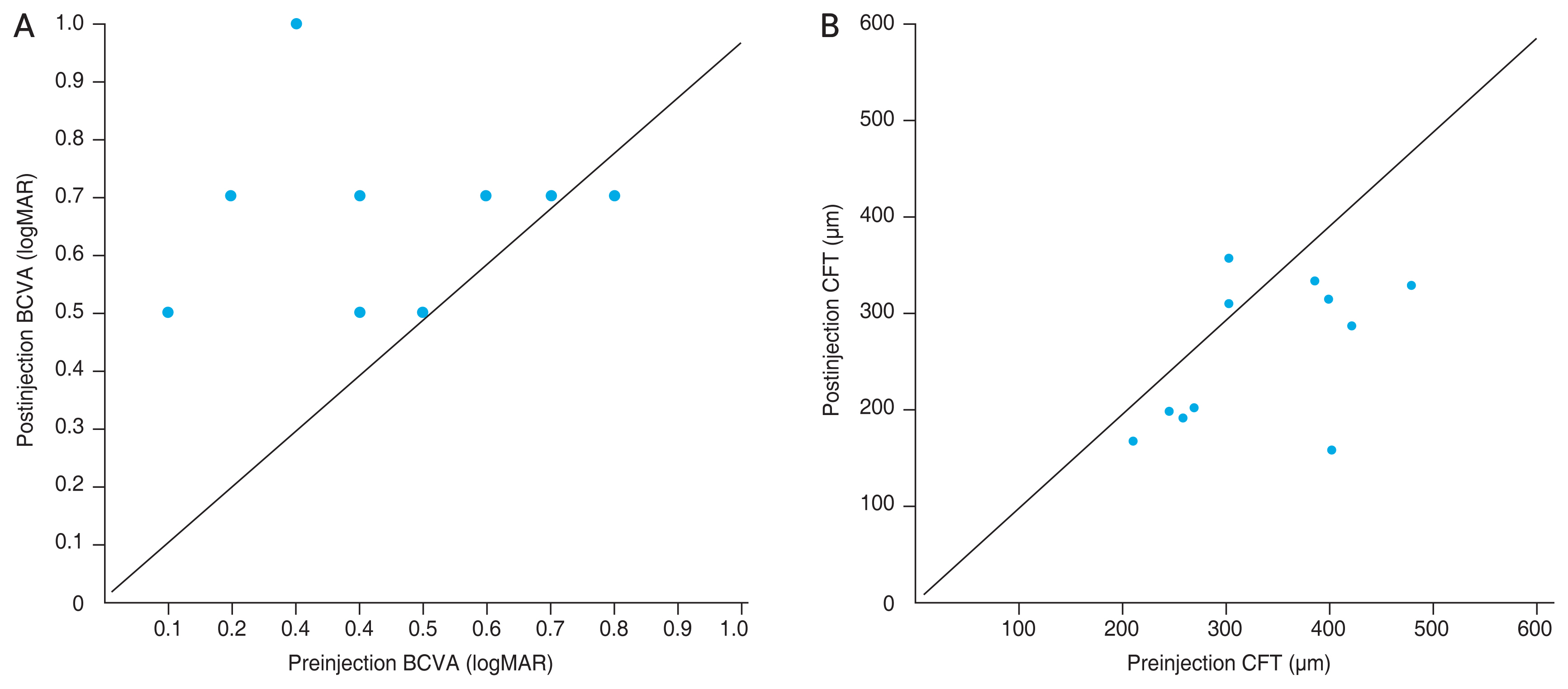
Fig. 2
Scatter plot of the concentration change of cytokines preinjection and postinjection. (A) Interleukin (IL)-6. (B) IL-8. (C) Interferon-induced protein (IP)-10. (D) Monocyte chemotactic protein (MCP)-1. (E) Platelet-derived growth factor (PDGF)-AA. (F) Vascular endothelial growth factor (VEGF).
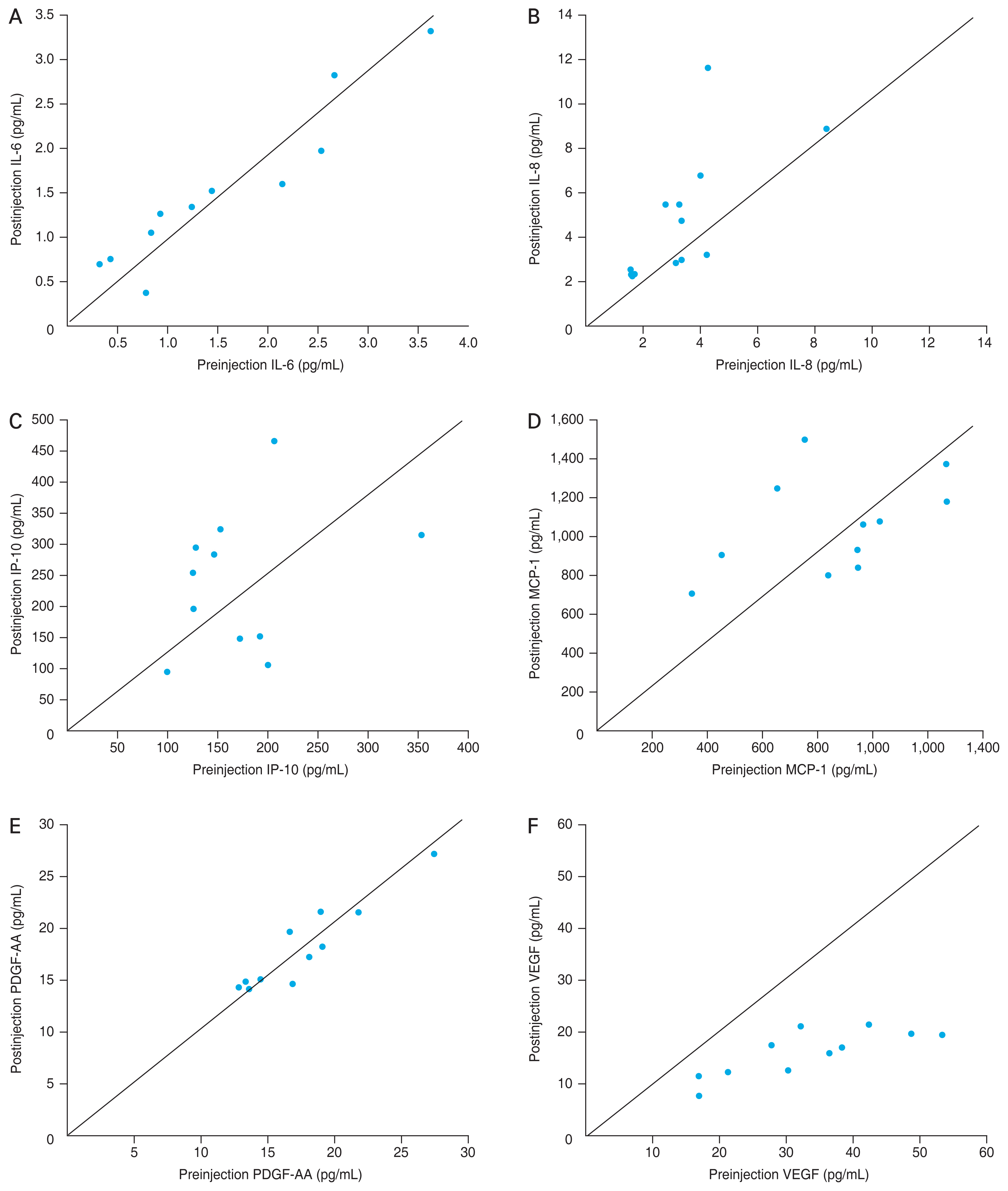
Table 1
Ophthalmologic findings of the patients (n = 12)
| Patient no. | Pigment epithelial detachment | Outer retinal layer or RPE irregularity | FA pattern | No. of bevacizumab* injection (relapse) |
|---|---|---|---|---|
| 1 | + | RPE hump | Smokestack | 4 |
| 2 | − | IS-OS disruption | Ink blot | 5 |
| 3 | − | IS-OS disruption | Diffuse | 4 |
| 4 | − | RPE defect | Diffuse | 4 |
| 5 | + | RPE hump | Ink blot | 6 |
| 6 | − | IS-OS disruption | Smokestack | 11 |
| 7 | + | RPE hump | Smokestack | 11 |
| 8 | + | RPE hump | Diffuse | 5 |
| 9 | − | IS-OS disruption | Diffuse | 6 |
| 10 | + | RPE hump, IS-OS disruption | Diffuse | 5 |
| 11 | − | IS-OS disruption | Ink blot | 4 |
| 12 | − | IS-OS disruption | Diffuse | 4 |
Table 2
Baseline characteristics of the patients (n = 18)
| Characteristic | CSC group (n = 12) | Control group (n = 6) |
|---|---|---|
| Sex | ||
| Male | 10 | 4 |
| Female | 2 | 2 |
| Age (yr) | 48.7 ± 9.2 | 51.8 ± 5.7 |
| Hypertension | 1 | 1 |
| Duration of SRF (mon) | 7.7 ± 2.5 | - |
Table 3
Changes in the BCVA, IOP, and CFT of the control and CSC groups (n = 18)
| Variable | Control group (n = 6) | CSC group (n = 12) | ||
|---|---|---|---|---|
|
|
||||
| Preinjection | Postinjection | p-value | ||
| BCVA (logMAR) | 0.52 ± 0.15 | 0.43 ± 0.32 | 0.27 ± 0.24 | 0.066 |
| IOP (mmHg) | 12.0 ± 1.4 | 11.0 ± 1.2 | 10.6 ± 1.2 | 0.534 |
| CFT (μm) | - | 328.08 ± 84.23 | 283.91 ± 112.55 | 0.048* |
Table 4
Aqueous concentrations of inflammatory and angiogenic cytokines before the intravitreal injection in the control and CSC groups (n = 18)
| Cytokine | Control group (n = 6) | CSC group (n = 12) | p-value* |
|---|---|---|---|
| IL-6 (pg/mL) | 1.1 ± 1.0 (0.3-3.0) | 0.9 ± 1.1 (0.3-2.6) | 0.316 |
| IL-8 (pg/mL) | 2.9 ± 1.2 (1.5-4.5) | 3.3 ± 2.3 (1.5-8.3) | 0.517 |
| IP-10 (pg/mL) | 208.1 ± 103.6 (103.3-372.8) | 165.7 ± 95.7 (100.2-353.3) | 0.303 |
| MCP-1 (pg/mL) | 913.7 ± 345.8 (526.3-1,452.5) | 840.0 ± 338.2 (450.3-1,267.0) | 0.558 |
| PDGF-AA (pg/mL) | 20.5 ± 7.7 (12.7-32.5) | 18.1 ± 6.8 (12.8-27.4) | 0.153 |
| VEGF (pg/mL) | 30.4 ± 11.5 (14.2-46.8) | 31.4 ± 12.3 (17.0-53.3) | 0.631 |
Table 5
Changes in the aqueous concentrations of inflammatory and angiogenic cytokines after injection in the CSC group
| Cytokine | CSC group (n = 12) | p-value* | |
|---|---|---|---|
|
|
|||
| Preinjection | Postinjection | ||
| IL-6 (pg/mL) | 0.9 ± 1.1 (0.3-2.6) | 0.9 ± 1.4 (0.4-2.8) | 0.455 |
| IL-8 (pg/mL) | 3.3 ± 2.3 (1.5-8.3) | 4.7 ± 2.9 (2.2-11.6) | 0.046 |
| IP-10 (pg/mL) | 165.7 ± 95.7 (100.2-353.3) | 225.9 ± 117.1 (74.2-465.3) | 0.055 |
| MCP-1 (pg/mL) | 840.0 ± 338.2 (450.3-1,267.0) | 1,015.0 ± 342.5 (583.3-1,495.0) | 0.076 |
| PDGF-AA (pg/mL) | 18.1 ± 6.8 (12.8-27.4) | 18.4 ± 6.7 (12.7-27.1) | 0.339 |
| VEGF (pg/mL) | 31.4 ± 12.3 (17.0-53.3) | 15.2 ± 6.1 (7.7-21.5) | <0.010 |



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print