 |
 |
| Korean J Ophthalmol > Volume 37(5); 2023 > Article |
|
Abstract
Purpose
To evaluate changes of ocular surface dynamics using Keratograph 5M for 3 months after vitreoretinal surgery.
Methods
Eighty-three patients were divided into three groups: phacoemulsification group, vitrectomy group, and combined group. Keratograph 5M was performed for all patients at 1 week, 1 month, and 3 months after the surgery. Ocular surface dynamics parameters measured by Keratograph 5M, including noninvasive keratograph first tear film breakup time (NifBUT), noninvasive keratograph average tear film breakup time (NiaBUT), and tear meniscus height (TMH) were compared among the three groups over time.
Results
The mean age of all patients (46 men and 37 women) was 62.2 ┬▒ 8.4 years. NifBUT and NiaBUT were significantly decreased at 1 week after surgery compared to those at baseline in all three groups (all p < 0.001). NifBUT and NiaBUT in the phacoemulsification group almost recovered to the preoperative level, while those in the vitrectomy group and the combined group were still significantly less than those at baseline. NifBUT and NiaBUT in the phacoemulsification group were significantly longer than those in the vitrectomy group and the combined group at 3 months. After 1 week, TMHs were significantly higher in the vitrectomy group (p = 0.001) and the combined group (p = 0.022) than in the phacoemulsification group, while TMHs were significantly less in the vitrectomy group (p = 0.010) and the combined group (p < 0.001) than in the phacoemulsification group at 3 months after surgery.
Conclusions
These results suggest that vitreoretinal surgery could induce alteration of ocular surface dynamics for 3 months. The vitrectomy group and the combined group showed tear film instability compared to the cataract surgery alone group. Patients who underwent vitreoretinal surgery experienced more severe dry eye syndrome symptoms than those who underwent cataract surgery. Thus, managing dry eye syndrome after vitreoretinal surgery should be considered important for patients.
Vitreoretinal surgery is a type of ocular surgery to treat various morbidities of retina and vitreous body. Three-port vitrectomy is now considered a standard procedure of vitreoretinal surgery. After Kasner [1] introduced the first subtotal open-sky vitrectomy, a kind of primitive form of vitreoretinal surgery, in 1969, OŌĆÖMalley and Heintz [2] further described surgical development including differentiating into vitreous cutter, infusion cannula, and endoilluminator, resulting in the progress to the current three-port pars plana vitrectomy. Initially, vitrectomy with a 20-gauge port was applied for vitreoretinal disease patients. Later, sutureless vitrectomy using instrument with smaller gauge such as 23-, 25-, and 27-gauge was clinically performed [3-6]. In addition, combined vitreoretinal surgery with phacoemulsification is widely used.
Dry eye syndrome, also known as keratoconjunctivitis sicca, can show various ocular symptoms including fatigued eye, ocular irrigation, conjunctival redness, discharge formation, and blurred vision. Dry Eye Workshop has recently defined dry eye syndrome as a multifactorial disease that can induce loss of tear film homeostasis associated with instability of tear film, hyperosmolarity, inflammation or damage on ocular surface, and neurosensory abnormality [7]. Dry eye syndrome can be caused by various factors such as aging, altered hormonal secretion, environmental factors, usage of topical agents, systemic disease or systemic drug administration, autoimmune disease, reduced meibomian gland function, and previous surgical history [8]. Severe dry eye syndrome can cause prominent visual impairment, consequently reducing quality of life [9]. Therefore, it is important to preserve tear film stability clinically to improve quality of life and enhance patientŌĆÖs satisfaction.
To evaluate stability of tear film and ocular surface dynamics, tear film breakup time (TBUT) is widely used as a diagnostic tool. It measures the time of breaking a tear film after ocular surface staining using fluorescein dye under slit-lamp biomicroscopy [10]. However, Dry Eye Workshop recommends measuring noninvasive TBUT (NIBUT), because subjective estimating the fluorescein staining on ocular surface can increase intersessional or interpersonal variability and consequently reduce reproducibility [11].
Keratograph 5M (K5M; Oculus Optikger├żte GmbH) is an advanced topographer using Placido disc illumination, which can achieve objective measurement of NIBUT and tear meniscus height (TMH) under infrared (880 nm wavelength) without topical anesthesia, fluorescein staining or bright white illumination [12]. Hence, K5M has been suggested a diagnostic equipment for dry eye syndrome with favorable sensitivity and specificity and increased reproducibility [10].
Symptoms of dry eye syndrome for patients who have undergone a vitreoretinal surgery can be often overlooked easily, because retinal specialist might be more interested in retinal condition than in dry eye syndrome symptoms. Therefore, influence of vitreoretinal surgery on progression of dry eye syndrome and alteration of ocular surface dynamics has been investigated with various methods [13-17]. To the best of our knowledge, however, research evaluating consecutive changes of tear film after vitreoretinal surgery with an objective diagnostic tool have not been reported yet. Therefore, the objective of this study is to investigate changes of ocular surface dynamics using K5M for 3 months after vitrectomy, compared with other surgery groups including a phacoemulsification group and a combined surgery group.
This study was approved by the Institutional Review Board of Soonchunhyang University Cheonan Hospital (No. 2023-05-028). It adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all individual patients included in this study at enrolment.
This prospective observational case-control study was performed from March 2020 to February 2023. Patients who underwent pars plana vitrectomy and phacoemulsification were enrolled. Exclusion criteria were patients with existing ocular surface diseases, eyelid problems, nasolacrimal duct stenosis, or autoimmune diseases; those with a history of contact lens wearing, ocular trauma or other ophthalmic surgery; those who received combined scleral fixation of intraocular lens (IOL) or combined scleral buckling procedure with pars plana vitrectomy; and those who underwent another ophthalmic surgery additionally during the follow-up period.
Patients were divided into three groups: phacoemulsification (with IOL implantation) group, vitrectomy (pars plana vitrectomy alone) group, and combined (pars plana vitrectomy with phacoemulsification) group. All patients were followed up for 3 months after the surgery.
Phacoemulsification, vitrectomy, and combined surgery were performed by a single operator (HDK) for all patients in this study. In the beginning of all surgeries, periocular skin drape was performed using 10% povidone agent and conjunctival irrigation was done with 5% povidone solution.
When the phacoemulsif ication was performed, a phacoemulsifier device (Infiniti Ozil Intelligent Phaco, Alcon) was used and cataract extraction was performed through a clear corneal incision 2.2 mm of width. Topical mydriatic agent (Midrin P, Santen) was instilled before surgery. After topical anesthesia with 0.5% proparacaine hydrochloride (Alcaine, Alcon), a viscoelastic substance (sodium hyaluronate 1%, Hyalu, Hanmi Pharma Co) was injected into the anterior chamber through the corneal puncture site on 2 oŌĆÖclock area. Continuous curvilinear capsulorhexis was achieved using 26-gauge bent needle. After hydrodissection and phacoemulsification, hydrophobic acrylic foldable IOL (enVista, MX60, Bausch & Lomb) was implanted in the capsular bag. Corneal suture was not performed for all cataract patients. The surgery was finished after confirming the no leakage from the clear corneal incision site.
Vitrectomy and combined surgeries (pars plana vitrectomy with phacoemulsification) were performed with a standard three-port pars plana vitrectomy using a Stellaris PC device (Bausch & Lomb) with a 25-gauge trocar system under general or retrobulbar anesthesia. Cataract extraction and IOL implantation of combined surgery were performed with the same method as described above. The same hydrophobic acrylic foldable IOL was applied for vitrectomy and combined surgeries. Retina was visualized using a noncontact wide field viewing system (RESIGHT 700, Carl Zeiss Meditec), and corneal surface was maintained by intermittent application of ophthalmic a viscoelastic substance during the surgery. Superficial keratectomy or corneal peeling was not applied for vitrectomy or combined surgery cases. Vitrectomy and combined surgeries were finished after confirming no leakage at sclerotomy sites. Sclerotomy sites were approximated with absorbable suture materials. According to the retinal status, intravitreal gas tamponade was performed to recover retinal attachment.
After surgery, levofloxacin (Cravit1.5, Santen) and 1% prednisolone acetate (Predbell, Chong Kun Dang Pharmaceutical Corp) were instilled for patients four times a day. Homatropine (Homapine Ophthalmic Solution, Hanlim Pharma) was added for patients who underwent vitrectomy and combined surgery. For all patients, artificial tears and other ointments were not prescribed for 3 months after surgery. The operation time, defined as the time from corneal or scleral incision to the time of corneal or scleral wound closing, was measured by independent colleagues in operation room for three groups.
All patients who met the abovementioned criteria underwent comprehensive ophthalmic examinations, including best-corrected visual acuity with logarithm of the minimum angle of resolution units, intraocular pressure measurement using noncontact tonometry, slit-lamp biomicroscopy, and fundus examination. These ophthalmic examinations were performed for all patients before surgery and at each follow-up period after the surgery.
To evaluate ocular surface dynamics of patients after surgeries, NIBUT and TMH were estimated using K5M device four times for all patients: before surgery, and at 1 week, 1 month, and 3 months after surgery. The procedure was performed by skillful single technician without any other eyedrop instillation or irritation on ocular surface 1 hour prior to ocular surface dynamic evaluation.
When measuring the NIBUT, corneal surface was divided into 192 regions separated by eight circles and 24 radial straight lines after 22 concentric rings with infrared light were projected on the cornea by an embedded software. When the shape of the projected concentric ring on the corresponding region was changed, the NIBUT was recorded. Noninvasive keratograph first TBUT (Nif BUT) was the duration after blinking until the first tear film was destroyed. On the other hand, noninvasive keratograph average TBUT (NiaBUT) was calculated as the average duration until the tear film destruction time in all corneal regions (Fig. 1A).
When measuring the TMH, red-colored concentric ring illumination was disabled, and a dark background was applied for the patient. In the image obtained after blinking three times for 3 seconds, the vertical distance between lid margin and tear meniscus on the imaginary vertical line from the center of the cornea was recorded (Fig. 1B).
To compare categorical data among the three groups before the surgeries, Pearson chi-square test with Fisher exact test was used. One-way analysis of variation (ANOVA) with post hoc was used for continuous variables among three groups, and Mann-Whitney U-test was applied to compare the ocular surface dynamics parameters between two groups. Repeated measures ANOVA with Bonferroni correction was used to confirm that measurements had a significant difference from the baseline over time in each group after surgery. Pearson correlation test was conducted to analyze the correlations of the operation time and the measured values. All statistical analyses were performed using IBM SPSS ver. 26.0 (IBM Corp), and p-value less than 0.05 was considered statistically significant.
A total of 83 patients were enrolled in this study, and there were 46 men and 37 women. Their mean age was 62.2 ┬▒ 8.4 years. Table 1 presents the baseline characteristics of each group. In the phacoemulsification group, 31 patients (13 men, 18 women) underwent phacoemulsification surgeries. Their mean age was 67.74 ┬▒ 8.59 years. In the vitrectomy group, single vitrectomy was performed for 22 patients (14 men, 8 women). Their mean age was 64.54 ┬▒ 6.58 years. In the combined group, a total of 30 patients (19 men, 11 women) were included. Their mean age 63.20 ┬▒ 9.07 years. There was no significant difference in mean age (p = 0.098) or sex distribution (p = 0.162) among the three groups before surgery. The number of patients who had a history of diabetes mellitus was 14 in the phacoemulsification group, 9 in the vitrectomy group, and 11 in the combined group. There was no significant difference in the distribution of patients with a history of diabetes mellitus among the three groups (p = 0.797).
Etiology which required vitreoretinal surgery in the vitrectomy and combined groups included epiretinal membrane (ERM), vitreous hemorrhage by proliferative diabetic retinopathy (PDR), rhegmatogenous retinal detachment (RRD), and full-thickness macular hole (FTMH). In the vitrectomy group, eight patients underwent vitrectomy due to ERM, five patients had vitreous hemorrhage by PDR, eight patients showed RRD, and one patient had FTMH. On the other hand, 12 patients showed ERM, seven patients had vitreous hemorrhage caused by PDR, nine patients experienced RRD, and two patients showed FTMH. To attach the retina or seal the macular hole, intravitreal gas (sulfur hexafluoride) tamponade was performed for 12 patients in the vitrectomy group and 17 patients in the combined group.
Before surgery, average Nif BUT, NiaBUT, and TMH were 14.66 ┬▒ 1.36, 16.58 ┬▒ 2.03 seconds, and 0.28 ┬▒ 0.03 mm, respectively, in the phacoemulsification group; 15.02 ┬▒ 1.19, 17.56 ┬▒ 1.33 seconds, and 0.27 ┬▒ 0.03 mm, respectively, in the vitrectomy group; and 14.85 ┬▒ 1.33, 17.11 ┬▒ 1.59 seconds, and 0.28 ┬▒ 0.03 mm, respectively, in the combined group. There were no significant differences in preoperative Nif BUT ( p = 0.611), NiaBUT ( p = 0.125), or TMH (p = 0.406) among the three groups (Table 1).
As shown in Table 2, compared to baseline, NifBUTs at 1 week after surgery were significantly decreased in all three groups (all p < 0.001). In addition, postoperative NifBUTs at 1 month after surgery were significantly reduced compared to those at baseline in all three groups (all p Ōēż 0.001). At 3 months after surgery, Nif BUT in the phacoemulsification group was recovered almost to the preoperative level (p = 0.321), while those in the vitrectomy and combined groups were still significantly less than those at baseline (both p < 0.001).
When Nif BUTs were compared among three groups, NifBUT at 1 week after surgery in the phacoemulsification group was significantly longer than those in the vitrectomy group ( p = 0.036) and the combined group ( p = 0.001). NifBUTs in the phacoemulsification group were also significantly longer than those in the vitrectomy group and the combined group at 1 month (p = 0.001 and p < 0.001) and 3 months (p = 0.007 and p < 0.001) after surgery (Fig. 2). On the other hand, there was no significant difference in NifBUTs between the vitrectomy group and the combined group at 1 week ( p = 0.917), 1 month ( p = 0.095), or 3 months (p = 0.249) after surgery.
Alteration of postoperative NiaBUT compared to that at baseline showed similar pattern to NifBUT, at each postoperative time point (Table 2). NiaBUTs were significantly decreased compared to those at the baseline in all three groups at 1 week after surgery (all p < 0.001). NiaBUT in the phacoemulsification group was restored to near the baseline at 1 month (p = 0.081) and 3 months (p = 0.124) after surgery. Conversely, NiaBUT in the vitrectomy group decreased significantly at 1 month and 3 months after vitrectomy compared to that at the baseline (both p < 0.001). NiaBUT in the combined group was also significantly reduced at 1 month and 3 months after combined surgery (both p < 0.001).
NiaBUT at 1 week after the surgery in the phacoemulsification group was significantly longer than those in the vitrectomy group ( p = 0.005) and the combined group ( p < 0.001). NiaBUTs in the phacoemulsification group were also significantly longer than those in the vitrectomy group and combined group at 1 month ( p = 0.006 and p < 0.001) and 3 months ( p < 0.001 and p = 0.034) after surgery (Fig. 3). On the other hand, there was no significant difference in NiaBUT between the vitrectomy group and the combined group at 1 month (p = 0.311) or 3 months ( p = 0.353) after surgery. Alteration of NiaBUT in all groups revealed a similar tendency to NifBUT after surgery during the follow-up period.
As shown in Table 2, during the follow-up period, TMH in the phacoemulsification group revealed no significant difference at each time point (postoperative 1 week, p = 0.068; 1 month, p = 0.090; and 3 months, p = 0.154) from that at baseline. On the other hand, TMHs in the vitrectomy group were significantly decreased at postoperative 1 week (p = 0.004), 1 month (p = 0.017), and 3 months (p = 0.003). TMHs in the combined group at postoperative 1 week ( p = 0.042), 1 month ( p = 0.378), and 3 months (p < 0.001) were also significantly less than that at baseline.
At 1 week after surgery, TMHs were significantly higher in the vitrectomy group ( p = 0.001) and the combined group (p = 0.022) than in the phacoemulsification group (Fig. 4). At 1 month of surgery, TMHs were significantly reduced in the vitrectomy group compared to those in the phacoemulsification group (p = 0.029). At 3 months after surgery, TMHs in the vitrectomy group (p = 0.010) and the combined group ( p < 0.001) were significantly less than that in the phacoemulsification group. On the other hand, there was no significant difference in TMH between the vitrectomy group and the combined group at 1 week (p = 0.491), 1 month (p = 0.853), or 3 months (p = 0.685) after surgery during the follow-up period.
Further analysis was performed to determine whether diabetes of patients could affect the ocular surface dynamics measured by K5M after vitreoretinal surgery (Table 3). For patients with diabetes in the vitrectomy group, Nif-BUTs was 14.91 ┬▒ 0.85 seconds at baseline, 10.49 ┬▒ 2.98 seconds at 1 week, 12.68 ┬▒ 1.36 seconds at 1 month, and 12.30 ┬▒ 1.36 seconds at 3 months after surgery. For patients without diabetic history, NifBUTs was 15.09 ┬▒ 1.41 seconds at baseline, 10.46 ┬▒ 2.31 seconds at 1 week, 11.20 ┬▒ 2.86 seconds at 1 month, and 13.16 ┬▒ 2.12 seconds at 3 months after vitrectomy. There was no significant difference in NifBUT for the vitrectomy group at each time point according to diabetic history (baseline, p = 0.556; postoperative 1 week, p = 0.896; 1 month, p = 0.110; and 3 months, p=0.144). Mean NiaBUT and TMH also showed no differences either according to the presence of diabetes in the vitrectomy group. In the combined group, diabetic history of patients did not affect ocular surface dynamics after surgery either.
To attach the retina, intravitreal gas tamponade was performed before the end of surgery for 12 patients in the vitrectomy group and 17 patients in the combined groups. Additional analysis was performed to determine whether intravitreal gas tamponade could affect ocular surface dynamics parameters after vitreoretinal surgeries (Table 4). In the vitrectomy group, Nif BUT, NiaBUT and TMH showed no significant difference between tamponade and non-tamponade subgroups at 1 week, 1 month, or 3 months after the vitrectomy. In the combined group, whether intravitreal gas filling surgery was performed did not affect results of ocular surface dynamics such as Nif BUT, NiaBUT, or TMH after the surgery either.
The average operation times was 21.03 ┬▒ 4.58 minutes in the phacoemulsification group, 56.45 ┬▒ 10.63 minutes in the vitrectomy group, and 88.63 ┬▒ 28.10 minutes in the combined group. When comparing the three groups, the phacoemulsification group spent a significantly shorter operation time than the vitrectomy group (p < 0.001), and the combined group required significantly more time than the vitrectomy group (p < 0.001) (Table 1). When all three groups were analyzed together, the operation time has significant negative correlation with NifBUT, NiaBUT, and TMH at postoperative 1 month (r = ŌłÆ0.605, r = ŌłÆ0.588, and r = ŌłÆ0.274; p < 0.001, p < 0.001, and p = 0.012, respectively) and postoperative 3 months (r = ŌłÆ0.610, r = ŌłÆ0.376, and r = ŌłÆ0.579; all p < 0.001, respectively) (Table 5).
Several mechanisms have been suggested for changes of ocular surface dynamics and tear film status after a vitreoretinal surgery. Surgical procedure is considered as a kind of ocular surface trauma with consequent inflammatory reaction that can induce dry eye syndrome [18]. In addition, upregulation of inflammatory cytokines including interleukin-1╬▓ (IL-1╬▓), IL-6, IL-8, IL-1 receptor antagonist (IL-1RA), IL-5, IL-9, fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), IL-2, IL-6, IL-15, granulocyte macrophage colony-stimulating factor (GM-CSF), and interferon-╬│ (IFN-╬│) in tear fluid has been suggested [17,19]. Changes of conjunctival goblet cell density and aquaporin expression after vitreoretinal surgeries can induced dry eye syndrome [17,20]. A vitreoretinal surgery can reduce corneal sensation. It can consequently affect lid blinking of patient [21]. Moreover, secretion of lacrimal gland might decrease after surgical procedures [22].
Preoperative risk factors that can affect dry eye syndrome occurrence include patientŌĆÖs or ophthalmological conditions such as age, female sex, previous dry eye syndrome history, and a history of diabetic retinopathy [23-25]. In addition, longer exposure time to microscopic light can progress the symptom of dry eye syndrome after surgery [26]. Intraoperative trauma on corneal epithelium, conjunctival surface, eyelid, and lacrimal gland can also contribute to the development of dry eye syndrome [22-25,27]. After a vitreoretinal surgery, administration of eyedrops and suture materials at the sclerotomy site can induce dry eye syndrome [16,21].
This study was performed to objectively evaluate changes of ocular surface dynamics and dry eye parameters after vitreoretinal surgery using a K5M device. In this study, NiaBUT and NifBUT showed no significant alterations at 3 months after a single cataract surgery. However, they were significantly reduced at 3 months after vitrectomy or combined surgery. These results suggested that tear film stability of the corneal surface was decreased after vitrectomy during the follow-up period. The discrepancy between the cataract surgery group and the other groups might be due to suture materials at sclerotomy sites. Goblet cells in conjunctival tissue secretes mucin. Suturing on conjunctiva and tenon capsule could induce loss of goblet cell and consequently reduce mucin secretion, which might have affected recovery of the tear film on the ocular surface [26].
Postoperative TMH in this study was significantly increased at 1 week compared to those at baseline in the vitrectomy group and combined group. Making the scleral incision and the use of suture materials at the scleral incision sites might have contributed to an increase in the height of tear meniscus. Complete absorption of suture materials used at the sclerotomy site need approximated 56 to 70 days after surgery. It seems that suture materials caused ocular irritation and increased TMH transiently. Previously, Sato et al. [15] have also reported that TMH is significantly increased at 1 week after 25-gauge vitrectomy than that at baseline, and TMH was much higher in sclerotomy suturing group.
The vitrectomy group and the combined surgery group spent longer operation times than the phacoemulsification group in this study. All parameters of ocular surface dynamics including NiaBUT and NifBUT in the vitrectomy group and the combined group were deleterious at 3 months after surgery. Long operation time or extended exposure to the light might have influenced postoperative tear film stability. However, NiaBUT, NifBUT, and TMH showed no significant difference between the vitrectomy group and the combined group. In addition, NiaBUT, Nif-BUT, and TMH in the phacoemulsification group showed no significant change during the follow-up period, except at 1 week after surgery. These findings suggested that cataract surgery did not contribute remarkable effect on the development of dry eye syndrome in the combined group. It might be because manipulation on the ocular surface was minimized, noncontact type wide field viewing system was used, and viscoelastic substance was applied during surgery to protect the corneal surface. It might be because vitrectomy was performed under endoilluminator without light from microscope, and exposure to microscope light source could be avoided during the operation time. In this study, intravitreal gas tamponade did not affect postoperative changes of ocular surface dynamics parameters.
A previous study has reported that tear secretion is reduced in diabetic patients after surgery, which could worsen the symptoms of dry eye syndrome [28]. Vitreoretinal surgery was considered a risk factor for corneal complications including epithelial disturbance and corneal edema in diabetic patients [29]. Conversely, another study has shown that diabetes is not associated with dry eye syndrome signs or Ocular Surface Disease Index (OSDI) scores [30]. In this study, there were no statistically significant difference in parameters of ocular surface dynamics according to the diabetic history.
In previous reports on ocular surface changes after vitreoretinal surgery, Ghasemi et al. [13] have found that results of Schirmer test are decreased significantly at 1 month and 3 months after vitrectomy using 20- and 23-gauge trocar systems. They also found that results of Schirmer test at 3 months after vitrectomy in the 20-gauge group were significantly less than the 23-gauge group. Lee et al. [16] have analyzed effect of suturing on the sclerotomy sites in the 23-gauge vitrectomy at 1 week, 1 month, and 3 months after surgery. They reported that TBUT at 3 months after surgery was significantly longer, and OSDI was much lower in the sutureless vitrectomy group. Mani et al. [17] have also evaluated symptoms and signs of dry eye syndrome after a minimally invasive vitrectomy with scleral encircling. They found no significant change in clinical symptoms of patients, although the density of goblet cells in the conjunctival tissue was significantly reduced after vitrectomy. Furthermore, several inflammatory cytokines including IL-5, IL-9, FGF, PDGF, IL-2, and IL-6 in the tear fluid were increased, and expression levels associated with mucin and aquaporin were changed after vitrectomy.
This study has several limitations. First, the follow-up period of this study was only 3 months, which was relatively short. In addition, the number of subjects was small. Second, TBUT and TMH which were measured by K5M can be influenced by environmental factors such as age or sex. Third, other evaluating methods such as Schirmer test, corneal fluorescence staining score, and OSDI for dry eye syndrome were not combined with K5M measurement. Therefore, it was a limitation that signs of dry eye syndrome were not evaluated in various aspects before or after vitreoretinal surgery. Fourth, subjective symptoms of patients were not estimated quantitatively because a questionnaire for dry eye syndrome was not applied in this study. Fifth, suture materials in vitrectomy and combined surgery groups might have acted as a kind of bias in this study. To prevent wound leakage on sclerotomy sites, sclerotomy site suture was inevitably performed for patients who underwent vitrectomy and combined surgery, especially combined with intravitreal gas tamponade. In the future, changes of ocular surface dynamics and development of dry eye syndrome after sutureless vitrectomy need to be investigated.
However, changes of ocular surface dynamics and dry eye syndrome signs after vitreoretinal surgery were evaluated objectively using the K5M device in this study, and alteration of ocular surface dynamics over time after the vitreoretinal surgery was shown. In addition, this study was meaningful in that ocular surface dynamics parameters were compared to the single cataract surgery and combined surgery patients.
In conclusion, vitreoretinal surgery induced alteration of ocular surface dynamics. Tear film instability was revealed in vitrectomy and combined surgery groups, compared to the cataract surgery alone group. In addition, these deleterious findings of ocular surface dynamics maintained at 3 months after vitreoretinal surgery. These results might be caused by long operation time and suture materials at the sclerotomy sites. This study suggests that patients who undergo vitreoretinal surgery might experience more severe dry eye syndrome symptoms than cataract surgery patients. Thus, the management of dry eye syndrome after vitreoretinal surgery should be considered important. Further research for dry eye syndrome and ocular surface dynamics associated with vitreoretinal surgery with a larger number for a long period is needed in the future.
References
1. Kasner D. Vitrectomy: a new approach to management of vitreous. Highlights Ophthalmol 1969;11:304-29.
2. OŌĆÖMalley C, Heintz RM Sr. Vitrectomy with an alternative instrument system. Ann Ophthalmol 1975;7:585-8. 591-4.

3. Mason G, Sullivan JM, Olk RJ. A sutureless self-retaining infusion cannula for pars plana vitrectomy. Am J Ophthalmol 1990;110:577-8.

4. Peyman GA. A miniaturized vitrectomy system for vitreous and retinal biopsy. Can J Ophthalmol 1990;25:285-6.

5. Fujii GY, de Juan E Jr, Humayun MS. Improvements after sheathotomy for branch retinal vein occlusion documented by optical coherence tomography and scanning laser ophthalmoscope. Ophthalmic Surg Lasers Imaging 2003;34:49-52.


6. Oshima Y, Wakabayashi T, Sato T, et al. A 27-gauge instrument system for transconjunctival sutureless microincision vitrectomy surgery. Ophthalmology 2010;117:93-102.


7. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf 2017;15:276-83.


8. Han KE, Yoon SC, Ahn JM, et al. Evaluation of dry eye and meibomian gland dysfunction after cataract surgery. Am J Ophthalmol 2014;157:1144-50.


9. Shimazaki-Den S, Dogru M, Higa K, Shimazaki J. Symptoms, visual function, and mucin expression of eyes with tear film instability. Cornea 2013;32:1211-8.


10. Hong J, Sun X, Wei A, et al. Assessment of tear film stability in dry eye with a newly developed keratograph. Cornea 2013;32:716-21.


11. Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop 2007. Ocul Surf 2007;5:108-52.


12. Abdelfattah NS, Dastiridou A, Sadda SR, Lee OL. Noninvasive imaging of tear film dynamics in eyes with ocular surface disease. Cornea 2015;34:Suppl 10. S48-52.


13. Ghasemi FK, Shaheen Y, Karimi MA, et al. Schirmer test changes after 20 gauge and 23 gauge pars plana vitrectomy. Rom J Ophthalmol 2017;61:39-43.



14. Nemcansky J, Kopecky A, Masek P. Changes in tear film osmolarity after 25G+ PPV. BMC Ophthalmol 2020;20:452.


15. Sato T, Koh S, Yasukura YI, et al. Surgical factors affecting changes in ocular surface dynamics in the early postoperative period after 25-gauge vitrectomy. Eye Contact Lens 2019;45:254-9.


16. Lee JH, Na KS, Kim TK, et al. Effects on ocular discomfort and tear film dynamics of suturing 23-gauge pars plana vitrectomies. Arq Bras Oftalmol 2019;82:214-9.


17. Mani R, Shobha PS, Thilagavathi S, et al. Altered mucins and aquaporins indicate dry eye outcome in patients undergoing Vitreo-retinal surgery. PLoS One 2020;15:e0233517.



18. Wei Y, Asbell PA. The core mechanism of dry eye disease is inflammation. Eye Contact Lens 2014;40:248-56.



19. Fujita A, Uchino E, Otsuka H, et al. Ocular surface molecule after transconjunctival vitrectomy. Br J Ophthalmol 2011;95:419-23.


20. Heimann H, Coupland SE, Gochman R, et al. Alterations in expression of mucin, tenascin-c and syndecan-1 in the conjunctiva following retinal surgery and plaque radiotherapy. Graefes Arch Clin Exp Ophthalmol 2001;239:488-95.



21. Khanal S, Tomlinson A, Esakowitz L, et al. Changes in corneal sensitivity and tear physiology after phacoemulsification. Ophthalmic Physiol Opt 2008;28:127-34.


22. Stern ME, Beuerman RW, Fox RI, et al. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea 1998;17:584-9.


23. Perry HD, Donnenfeld ED. Dry eye diagnosis and management in 2004. Curr Opin Ophthalmol 2004;15:299-304.


24. Ram J, Gupta A, Brar G, et al. Outcomes of phacoemulsification in patients with dry eye. J Cataract Refract Surg 2002;28:1386-9.


25. Chen WL, Lin CT, Ko PS, et al. In vivo confocal microscopic findings of corneal wound healing after corneal epithelial debridement in diabetic vitrectomy. Ophthalmology 2009;116:1038-47.


26. Li XM, Hu L, Hu J, Wang W. Investigation of dry eye disease and analysis of the pathogenic factors in patients after cataract surgery. Cornea 2007;26(9 Suppl 1):S16-20.


27. Cho YK, Kim MS. Dry eye after cataract surgery and associated intraoperative risk factors. Korean J Ophthalmol 2009;23:65-73.



28. Liu X, Gu YS, Xu YS. Changes of tear film and tear secretion after phacoemulsification in diabetic patients. J Zhejiang Univ Sci B 2008;9:324-8.




Fig.┬Ā1
Representative findings for ocular surface dynamics using Keratograph 5M (Oculus Optikger├żte GmbH) for patients. (A) Noninvasive tear breakup time (NIBUT) is obtained with an embedded software after projecting concentric rings on the cornea surface with infrared light. NIBUT was measured when the shape of the concentric ring changes in each region. (B) Tear meniscus height was measured using Keratograph 5M as vertical distance between the lower eyelid border and the center of the pupil from the image after the last blinking.
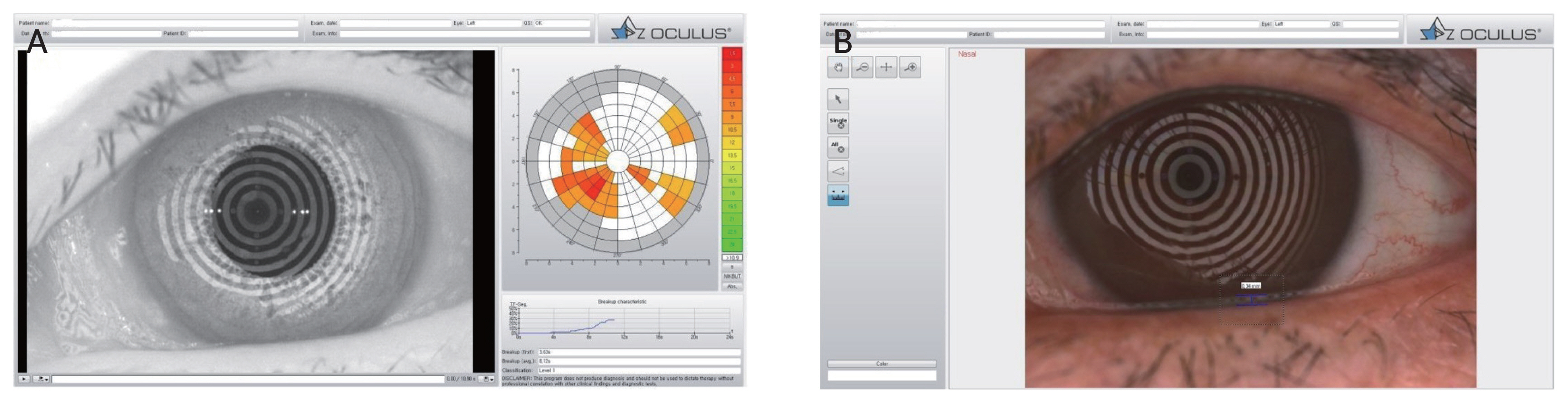
Fig.┬Ā2
Consecutive changes of noninvasive keratograph first tear film breakup time (NifBUT) in three groups. The mean NifBUT in the phacoemulsification group was significantly longer than those in the vitrectomy group and the combined group at 1 week, 1 month, and 3 months after surgery, respectively. On the other hand, there was no significant difference in Nif BUT between the vitrectomy group and the combined group at 3 months after surgery. Error bar indicates standard error of the mean. *At each follow-up period, p < 0.05.
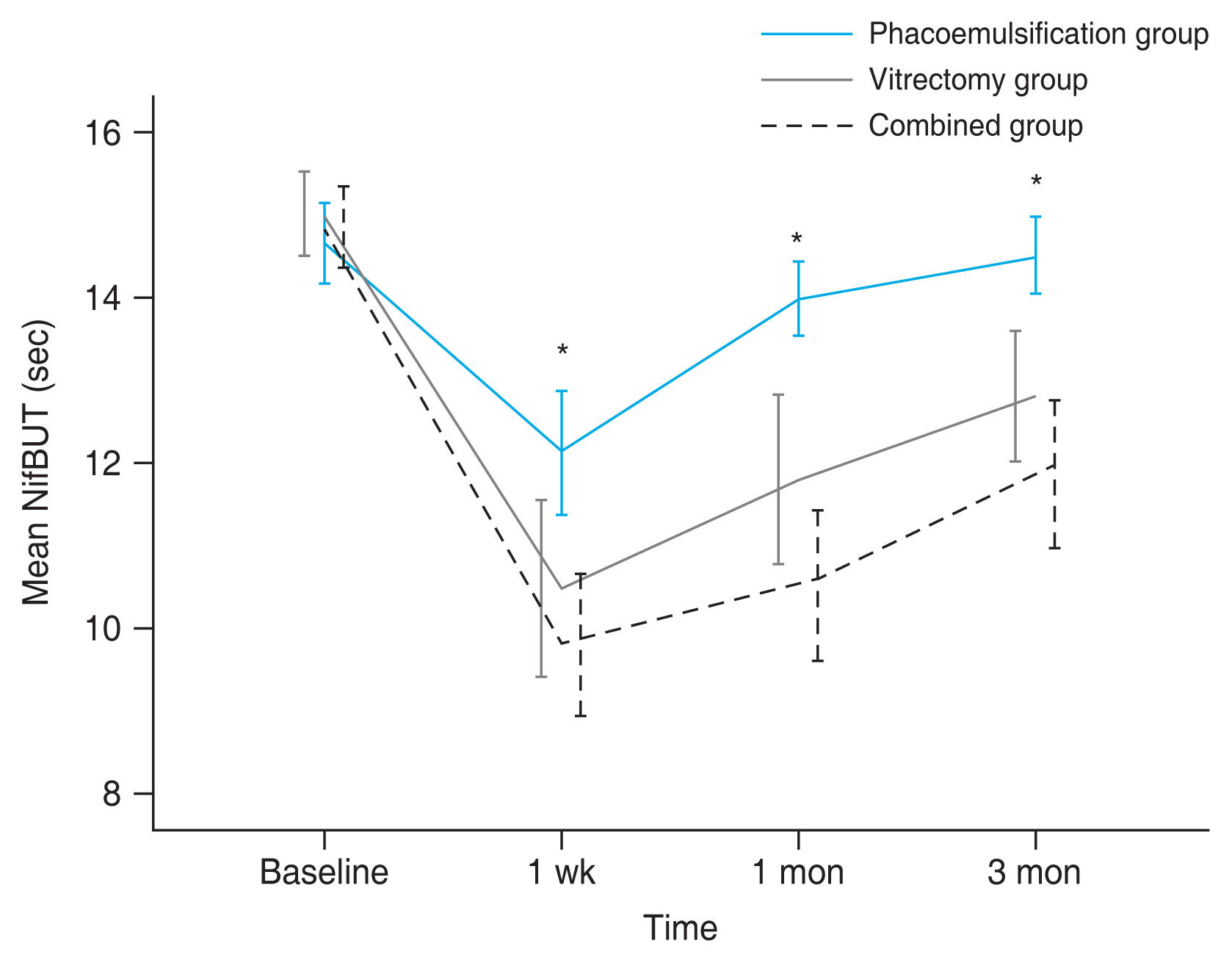
Fig.┬Ā3
Consecutive changes of noninvasive keratograph average tear film breakup time (NiaBUT) in three groups. The mean NiaBUT in the phacoemulsification group was significantly longer than those in the vitrectomy group and the combined group at 1 week, 1 month, and 3 months after the surgery. On the other hand, there was no significant difference in Nif BUT between the vitrectomy group and the combined group at 1 month and 3 months after surgeries. Error bar indicates standard error of the mean. *At each follow-up period, p < 0.05.

Fig.┬Ā4
Consecutive changes of tear meniscus height (TMH) in three groups. Mean TMHs after 1 week were significantly higher in the vitrectomy group (p = 0.001) and the combined group (p = 0.022) than in the phacoemulsification group (asterisk). At 1 month after surgery, TMHs in the vitrectomy group were significantly reduced, compared to those in the phacoemulsification group (p = 0.029, dagger). At 3 months after surgery, TMHs in the vitrectomy group (p = 0.010) and the combined group (p < 0.001) were significantly less than that in the phacoemulsification group (asterisk). On the other hand, there was no significant difference in TMH between the vitrectomy group and the combined group for 3 months. Error bar indicates standard error of the mean.

Table┬Ā1
Demographic and clinical characteristics of three groups at baseline (n = 83)
| Characteristic | Phacoemulsification group (n = 31) | Vitrectomy group (n = 22) | Combined group (n = 30) | p-value |
|---|---|---|---|---|
| Female sex | 18 (58.1) | 8 (36.4) | 11 (36.7) | 0.162* |
| Age (yr) | 67.74 ┬▒ 8.59 | 64.54 ┬▒ 6.58 | 63.20 ┬▒ 9.07 | 0.098ŌĆĀ |
| Laterality | 0.340* | |||
| ŌĆāRight eye | 15 (48.4) | 15 (68.2) | 18 (60.0) | |
| ŌĆāLeft eye | 16 (51.6) | 7 (31.8) | 12 (40.0) | |
| Diabetes history | 14 (45.2) | 9 (40.9) | 11 (36.7) | 0.797* |
| Operation time (min) | 21.03 ┬▒ 4.58 | 56.45 ┬▒ 10.63 | 88.63 ┬▒ 28.10 | <0.001ŌĆĀ |
| NifBUT (sec) | 14.66 ┬▒ 1.36 | 15.02 ┬▒ 1.19 | 14.85 ┬▒ 1.33 | 0.611ŌĆĀ |
| NiaBUT (sec) | 16.58 ┬▒ 2.03 | 17.56 ┬▒ 1.33 | 17.11 ┬▒ 1.59 | 0.125ŌĆĀ |
| TMH (mm) | 0.28 ┬▒ 0.03 | 0.27 ┬▒ 0.03 | 0.28 ┬▒ 0.03 | 0.406ŌĆĀ |
Table┬Ā2
Ocular surface dynamics parameters measured before surgery and at 1 week, 1 month, and 3 months after surgery in three groups
| Parameter | Baseline | Postoperative | p-value* | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| 1 wk | 1 mon | 3 mon | Overall | Baseline vs. 1 wk | Baseline vs. 1 mon | Baseline vs. 3 mon | ||
| Phacoemulsification group | ||||||||
| ŌĆāNifBUT (sec) | 14.66 ┬▒ 1.36 | 12.13 ┬▒ 2.07 | 13.99 ┬▒ 1.25 | 14.50 ┬▒ 1.29 | <0.001 | <0.001 | 0.001 | 0.321 |
| ŌĆāNiaBUT (sec) | 16.58 ┬▒ 2.03 | 14.52 ┬▒ 1.73 | 15.51 ┬▒1.85 | 15.50 ┬▒ 2.75 | <0.001 | <0.001 | 0.081 | 0.124 |
| ŌĆāTMH (mm) | 0.28 ┬▒ 0.03 | 0.27 ┬▒ 0.02 | 0.27 ┬▒ 0.03 | 0.27 ┬▒ 0.03 | <0.001 | 0.068 | 0.090 | 0.154 |
| Vitrectomy group | ||||||||
| ŌĆāNifBUT (sec) | 15.02 ┬▒ 1.19 | 10.47 ┬▒ 2.54 | 11.81 ┬▒ 2.44 | 12.81 ┬▒ 1.86 | <0.001 | <0.001 | <0.001 | <0.001 |
| ŌĆāNiaBUT (sec) | 17.56 ┬▒ 1.33 | 12.77 ┬▒ 1.99 | 13.52 ┬▒ 1.94 | 12.64 ┬▒ 2.79 | <0.001 | <0.001 | <0.001 | <0.001 |
| ŌĆāTMH (mm) | 0.27 ┬▒ 0.03 | 0.31 ┬▒ 0.04 | 0.24 ┬▒ 0.03 | 0.23 ┬▒ 0.04 | <0.001 | 0.004 | 0.017 | 0.003 |
| Combined group | ||||||||
| ŌĆāNifBUT (sec) | 14.85 ┬▒ 1.33 | 9.80 ┬▒ 2.37 | 10.52 ┬▒ 2.48 | 11.85 ┬▒ 2.46 | <0.001 | <0.001 | <0.001 | <0.001 |
| ŌĆāNiaBUT (sec) | 17.10 ┬▒ 1.59 | 11.28 ┬▒ 2.04 | 12.50 ┬▒ 2.67 | 13.79 ┬▒ 2.22 | <0.001 | <0.001 | <0.001 | <0.001 |
| ŌĆāTMH (mm) | 0.28 ┬▒ 0.03 | 0.30 ┬▒ 0.03 | 0.25 ┬▒ 0.04 | 0.21 ┬▒ 0.05 | <0.001 | 0.042 | 0.378 | <0.001 |
Table┬Ā3
Ocular surface dynamics parameters of patients with or without DM before surgery and at 1 week, 1 month, and 3 months after surgery in the vitrectomy group and the combined group
| Parameter | Baseline | Postoperative | ||
|---|---|---|---|---|
|
|
||||
| 1 wk | 1 mon | 3 mon | ||
| Vitrectomy group | ||||
| ŌĆāNifBUT (sec) | ||||
| ŌĆāŌĆāDM (+) | 14.91 ┬▒ 0.85 | 10.49 ┬▒ 2.98 | 12.68 ┬▒ 1.36 | 12.30 ┬▒ 1.36 |
| ŌĆāŌĆāDM (ŌłÆ) | 15.09 ┬▒ 1.41 | 10.46 ┬▒ 2.31 | 11.20 ┬▒ 2.86 | 13.16 ┬▒ 2.12 |
| ŌĆāŌĆāp-value* | 0.556 | 0.896 | 0.110 | 0.144 |
| ŌĆāNiaBUT (sec) | ||||
| ŌĆāŌĆāDM (+) | 17.59 ┬▒ 1.40 | 12.16 ┬▒ 1.24 | 12.77 ┬▒ 2.23 | 12.31 ┬▒ 2.72 |
| ŌĆāŌĆāDM (ŌłÆ) | 17.55 ┬▒ 1.35 | 13.20 ┬▒ 14.04 | 14.04 ┬▒ 1.59 | 12.86 ┬▒ 2.92 |
| ŌĆāŌĆāp-value* | 0.948 | 0.126 | 0.126 | 0.794 |
| ŌĆāTMH (mm) | ||||
| ŌĆāŌĆāDM (+) | 0.28 ┬▒ 0.03 | 0.33 ┬▒ 0.06 | 0.23 ┬▒ 0.04 | 0.23 ┬▒ 0.06 |
| ŌĆāŌĆāDM (ŌłÆ) | 0.27 ┬▒ 0.03 | 0.30 ┬▒ 0.02 | 0.25 ┬▒ 0.03 | 0.23 ┬▒ 0.03 |
| ŌĆāŌĆāp-value* | 0.948 | 0.471 | 0.471 | 0.794 |
| Combined group | ||||
| ŌĆāNifBUT (sec) | ||||
| ŌĆāŌĆāDM (+) | 14.93 ┬▒ 1.89 | 9.59 ┬▒ 2.03 | 10.95 ┬▒ 2.30 | 11.26 ┬▒ 2.55 |
| ŌĆāŌĆāDM (ŌłÆ) | 14.80 ┬▒ 0.83 | 9.94 ┬▒ 2.61 | 10.23 ┬▒ 2.26 | 12.25 ┬▒ 2.38 |
| ŌĆāŌĆāp-value* | 0.983 | 0.819 | 0.267 | 0.368 |
| ŌĆāNiaBUT (sec) | ||||
| ŌĆāŌĆāDM (+) | 17.14 ┬▒ 1.31 | 11.00 ┬▒ 2.32 | 12.56 ┬▒ 2.62 | 14.23 ┬▒ 2.13 |
| ŌĆāŌĆāDM (ŌłÆ) | 17.08 ┬▒ 1.79 | 11.46 ┬▒ 1.87 | 12.47 ┬▒ 2.77 | 13.49 ┬▒ 2.30 |
| ŌĆāŌĆāp-value* | 0.917 | 0.692 | 0.884 | 0.632 |
| ŌĆāTMH (mm) | ||||
| ŌĆāŌĆāDM (+) | 0.27 ┬▒ 0.04 | 0.30 ┬▒ 0.04 | 0.24 ┬▒ 0.05 | 0.19 ┬▒ 0.06 |
| ŌĆāŌĆāDM (ŌłÆ) | 0.28 ┬▒ 0.02 | 0.30 ┬▒0.03 | 0.26 ┬▒ 0.04 | 0.22 ┬▒ 0.04 |
| ŌĆāŌĆāp-value* | 0.491 | 0.602 | 0.415 | 0.232 |
Table┬Ā4
Ocular surface dynamics parameters of patients with or without intravitreal gas tamponade after surgery in the vitrectomy group and the combined group
| Parameter | Baseline | Postoperative | ||
|---|---|---|---|---|
|
|
||||
| 1 wk | 1 mon | 3 mon | ||
| Vitrectomy group | ||||
| ŌĆāNifBUT (sec) | ||||
| ŌĆāŌĆāTamponade (+) | 14.66 ┬▒ 1.23 | 10.98 ┬▒ 2.65 | 11.17 ┬▒ 2.49 | 12.57 ┬▒ 2.27 |
| ŌĆāŌĆāTamponade (ŌłÆ) | 15.53 ┬▒ 9.73 | 9.73 ┬▒ 2.30 | 12.72 ┬▒ 2.16 | 13.15 ┬▒ 1.06 |
| ŌĆāŌĆāp-value* | 0.071 | 0.262 | 0.235 | 0.471 |
| ŌĆāNiaBUT (sec) | ||||
| ŌĆāŌĆāTamponade (+) | 17.71 ┬▒ 1.47 | 12.19 ┬▒ 2.14 | 13.66 ┬▒ 1.72 | 13.27 ┬▒ 2.97 |
| ŌĆāŌĆāTamponade (ŌłÆ) | 17.35 ┬▒ 1.17 | 13.62 ┬▒ 1.47 | 13.33 ┬▒ 2.31 | 11.72 ┬▒ 2.36 |
| ŌĆāŌĆāp-value* | 0.647 | 0.209 | 0.896 | 0.110 |
| ŌĆāTMH (mm) | ||||
| ŌĆāŌĆāTamponade (+) | 0.28 ┬▒ 0.03 | 0.30 ┬▒ 0.04 | 0.23 ┬▒ 0.03 | 0.25 ┬▒ 0.05 |
| ŌĆāŌĆāTamponade (ŌłÆ) | 0.27 ┬▒ 0.04 | 0.33 ┬▒ 0.05 | 0.26 ┬▒ 0.03 | 0.23 ┬▒ 0.02 |
| ŌĆāŌĆāp-value* | 0.601 | 0.186 | 0.071 | 0.601 |
| Combined group | ||||
| ŌĆāNifBUT (sec) | ||||
| ŌĆāŌĆāTamponade (+) | 15.15 ┬▒ 1.24 | 10.39 ┬▒ 2.79 | 10.98 ┬▒ 2.77 | 12.60 ┬▒ 2.27 |
| ŌĆāŌĆāTamponade (ŌłÆ) | 14.46 ┬▒ 1.38 | 9.03 ┬▒ 1.42 | 9.93 ┬▒ 1.98 | 10.88 ┬▒ 2.43 |
| ŌĆāŌĆāp-value* | 0.113 | 0.281 | 0.385 | 0.086 |
| ŌĆāNiaBUT (sec) | ||||
| ŌĆāŌĆāTamponade (+) | 17.13 ┬▒ 1.82 | 11.34 ┬▒ 2.11 | 13.23 ┬▒ 2.91 | 13.89 ┬▒ 1.83 |
| ŌĆāŌĆāTamponade (ŌłÆ) | 17.08 ┬▒ 1.30 | 11.20 ┬▒ 2.02 | 11.55 ┬▒ 2.04 | 13.65 ┬▒ 2.73 |
| ŌĆāŌĆāp-value* | 0.742 | 0.902 | 0.086 | 0.967 |
| ŌĆāTMH (mm) | ||||
| ŌĆāŌĆāTamponade (+) | 0.29 ┬▒ 0.02 | 0.31 ┬▒ 0.02 | 0.27 ┬▒ 0.03 | 0.22 ┬▒ 0.05 |
| ŌĆāŌĆāTamponade (ŌłÆ) | 0.26 ┬▒ 0.03 | 0.28 ┬▒ 0.03 | 0.24 ┬▒ 0.05 | 0.20 ┬▒ 0.06 |
| ŌĆāŌĆāp-value* | 0.157 | 0.079 | 0.103 | 0.213 |
Table┬Ā5
Correlations of the operation time and ocular surface dynamics parameters
- TOOLS



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




