Comparative Evaluation of Matrix Metalloproteinase-9 Immunoassay and Tear Osmolarity Measurement for Diagnosing Severity of Dry Eye Disease
Article information
Abstract
Purpose
To evaluate and compare the clinical efficacy of matrix metalloproteinase-9 (MMP-9) immunoassay and tear osmolarity measurement in diagnosing dry eye severity.
Methods
Dry eye disease (DED) patients underwent diagnostic tests including MMP-9 assay, tear osmolarity measurement, fluorescein tear breakup time, ocular surface staining, anesthetized Schirmer test, Ocular Surface Disease Index questionnaire, and slit-lamp examination. The dry eye parameters were compared according to positive MMP-9 status and increased tear osmolarity. The correlation between dry eye profiles and MMP-9 positivity and high tear osmolarity was also analyzed.
Results
Those who tested positive in MMP-9 immunoassay had significantly higher corneal fluorescein staining score and worse DED severity than those who tested negative. The intensity of MMP-9 positivity showed positive correlation with the corneal staining score and DED severity. However, DED patients with high tear osmolarity above 308 mOsm/L did not show significantly different dry eye signs and symptoms compared to those with lower tear osmolarity values. Tear osmolarity was associated with ocular surface staining score in severe DED patients.
Conclusions
MMP-9 positivity was associated with ocular surface staining and worse dry eye severity. Therefore, it may be used as a useful indicator of disease severity in conjunction to other diagnostic tests.
Dry eye disease (DED) is a multifactorial condition characterized by symptoms such as discomfort, dryness, and visual disturbance, resulting from an imbalance in the tear film and the ocular surface. Tear hyperosmolarity, caused by the decreased tear volume and increased evaporation, is considered to be the trigger for an inflammatory cascade that leads to ocular surface epithelial damage and the release of proinflammatory cytokines and proteases. The inflammatory mediators exacerbate the ocular surface damage, destabilize the tear film, and perpetuates the vicious cycle of DED [1].
Inflammation is known to play a key role in the pathogenesis of dry eye. Increased levels of proinflammatory cytokines and matrix metalloproteinases (MMP) have been detected on the ocular surface of patients with DED [2]. Tear hyperosmolarity observed in DED has been shown to trigger the expression of inflammatory cytokines (interleukin-1β, tumor necrosis factor α) and MMP-9, which in turn activate the mitogen-activated protein kinase inflammatory cascade [3]. This cascade further promotes cytokine release, corneal epithelial damage, and goblet cell loss, exacerbating tear film instability and driving the self-perpetuating vicious cycle [4]. Therefore, inflammation and tear hyperosmolarity are recognized as key factors in the pathogenesis of dry eye and their detection on the ocular surface have been proposed as important diagnostic tests for DED.
MMP-9 is an early marker of inflammation which has been shown to be stimulated by desiccating stress [5]. T cell recruitment, the proteolytic activity of MMP-9 itself, and the stimulation of additional cytokine secretion triggers a self-perpetuating cycle of inflammation, secretory dysfunction, ocular surface damage, and aggravating eye dryness [4]. Its concentration and activity in the tear fluid of DED patients showed significant correlation with symptom severity scores and ocular signs such as tear breakup time (TBUT) and corneal and conjunctival fluorescein staining [6]. Using direct sampling microfiltration technology, the InflammaDry (Quidel Corp) immunoassay detects elevated levels of MMP-9 of more than 40 ng/mL in the tear fluid.
Since hyperosmotic stress has been shown to trigger the release of MMP-9 and plays a major role in initiating the cycle of progressive inflammation in DED [7], tear osmolarity measurement have been proposed as a useful diagnostic test for identifying patients with dry eye. Increased tear osmolarity have been found in patients with DED and intereye variability was suggested as a characteristic finding in DED patients [8].
The complex relationship between ocular surface inflammation and hyperosmotic stress are intertwined in the vicious cycle in that pathogenesis of DED. Therefore, this study was undertaken to compare the clinical efficacy of MMP-9 immunoassay and tear osmolarity osmometer in diagnosing DED and evaluating its severity.
Materials and Methods
Ethics statement
This study was approved by the Institutional Review Board of the National Health Insurance Service Ilsan Hospital (No. 2022-02-033). The requirement for informed consent was waived due to the retrospective nature of the study.
Patient selection
The records of patients who presented with dry eye symptoms were reviewed and those who satisfied the following criteria for DED were included in the study. Patients were included if they had either corneal fluorescein staining score of more than 1 (Oxford scheme), TBUT of less than 10 seconds, Schirmer I test of less than 10 mm/5 min, or Ocular Surface Disease Index (OSDI) of more than 12. Those with previous use of ocular medications other than artificial tears, contact lens use, and previous ocular surgery history within 6 months of visit were excluded. Patients were advised not to instill any eye drops 2 hours prior to the examination.
Tear osmolarity measurement
Tear osmolarity was measured using a tear osmometer, the TearLab Osmolarity System (TearLab Corp). The test card collects 50 nL of tear fluid from the lower tear meniscus. The reader then uses the electrical impedance to measure the osmolarity of the collected tear sample. The cutoff value for diagnosis of DED is suggested as 308 mOsm/L.
MMP-9 immunoassay
Tear samples were collected by gently dabbing the fleece of the sample collector on the patient’s lower palpebral conjunctiva. The sample collector is then placed within the test cassette and the absorbent tip is immersed into the buffer solution for more than 20 seconds. The cassette is laid flat for 10 minutes and the test results are interpreted. The presence of one blue line and one red line in the test window indicates a positive result of MMP-9 >40 ng/mL. The results were further defined as negative, equivocal, weak positive, positive, and strong positive, according to the intensity of the red line. The study testing was done on the subject’s more symptomatic eye. If there was no difference, the right eye was tested.
Clinical assessment
Clinical assessment was performed by one practitioner. A fluorescein strip was moistened and applied to inferior fornix. The patient was asked to blink several times and then keep their eyes open while the practitioner examined the patient using a slit-lamp microscope. TBUT was measured as the interval between the last blink and the first appearance of a dry spot on the corneal surface. Corneal and conjunctival staining was then examined and graded according to the Oxford scheme. Meibomian gland assessment was performed by applying a firm digital pressure to the central five glands of the lower lid. Meibum expressibility was graded on a scale of 0 to 3 (0, all five glands; 1, three to four glands; 2, one to two glands; 3, 0 glands) and meibum quality on a scale of 0 to 3 (0, clear; 1, cloudy; 2, granular; 3, inspissated). Then topical anesthetics were applied and Schirmer I test was performed. The test strip was placed on the lower lid margin for 5 minutes, and the amount of wetting was recorded. The patients completed the OSDI questionnaire for the assessment of their dry eye symptoms. Based on the results of the ocular examination, dry eye severity was graded according to Korean Corneal Disease Group guidelines for the diagnosis of DED [9].
Statistical analysis
Statistical analysis was conducted using IBM SPSS ver. 21 (IBM Corp). A t-test or Mann-Whitney U-test was used to analyze the difference between those who tested positive in the MMP-9 immunoassay and those who tested negative, and the difference between those who showed high tear osmolarity and those who did not. Partial correlation analysis was performed to investigate the association between the intensity of the positive result on MMP-9 assay and the results of other diagnostic tests for dry eye after adjusting for age and sex. The correlation was also evaluated for the association between tear osmolarity and other dry eye parameters. Subgroup analysis was performed for those with severe DED (TBUT ≤5 seconds, Schirmer I test <10 mm/5 min, and OSDI >12), aqueous deficiency dry eye (ADDE; Schirmer I test <10mm) and evaporative dry eye (EDE; meibum quality and expressibility grade ≥1 and TBUT ≤5 seconds).
Results
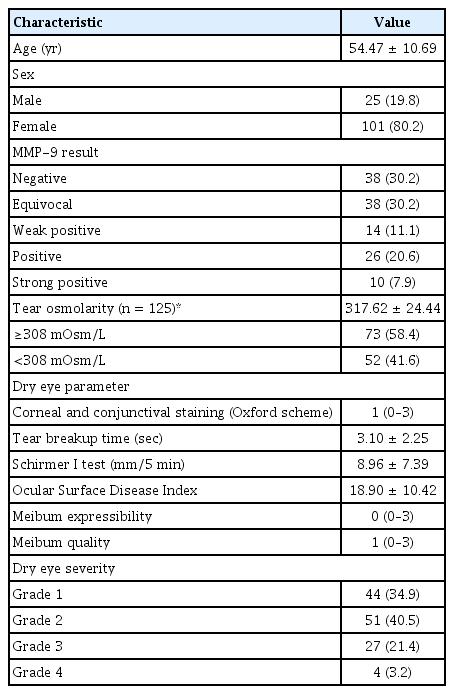
A total of 126 DED patients were included in the study, and 101 (80.2%) were female. The mean age of the subjects was 54.47 ± 10.69 years. Of 126 subjects, 88 (69.8%) tested positive for MMP-9 for InflammaDry testing. The demographics and clinical characteristics of the study subjects are shown in Table 1. Those who tested positive for MMP-9 showed significantly higher corneal and conjunctival fluorescein staining score than those who tested negative (Table 2). They also showed worse DED severity compared to those who tested negative. There were no significant differences in tear osmolarity, Schirmer I test score, OSDI, and meibomian gland assessment score between the two groups.
When the MMP-9 positive results were subdivided according to the intensity of the positive red line and the association of these results were analyzed with other dry eye parameters, the stronger intensity of the positive result, indicating higher MMP-9 level, was associated with higher corneal and conjunctival score (r = 0.334, p < 0.001), and worse dry eye severity (r = 0.387, p < 0.001). The association between the intensity of the MMP-9 positive result and other dry eye test results were not significant (TBUT: r = −0.142, p = 0.115; Schirmer I test: r = −0.132, p = 0.144; OSDI: r = −0.056, p = 0.537; meibum expressibility: r = 0.122, p = 0.179; meibum quality: r = 0.048, p = 0.595; tear osmolarity: r = −0079, p = 0.387). Fig. 1 shows the distribution of corneal and conjunctival fluorescein staining score according to the intensity of the positive MMP-9 immunoassay results. Those who show MMP-9 positive results with higher intensity show higher proportion of worse ocular surface staining score. Fig. 2 shows the distribution of dry eye severity grades according to the intensity of the positive MMP-9 immunoassay results. It shows that those with higher MMP-9 levels exhibit higher proportion of worse DED severity.
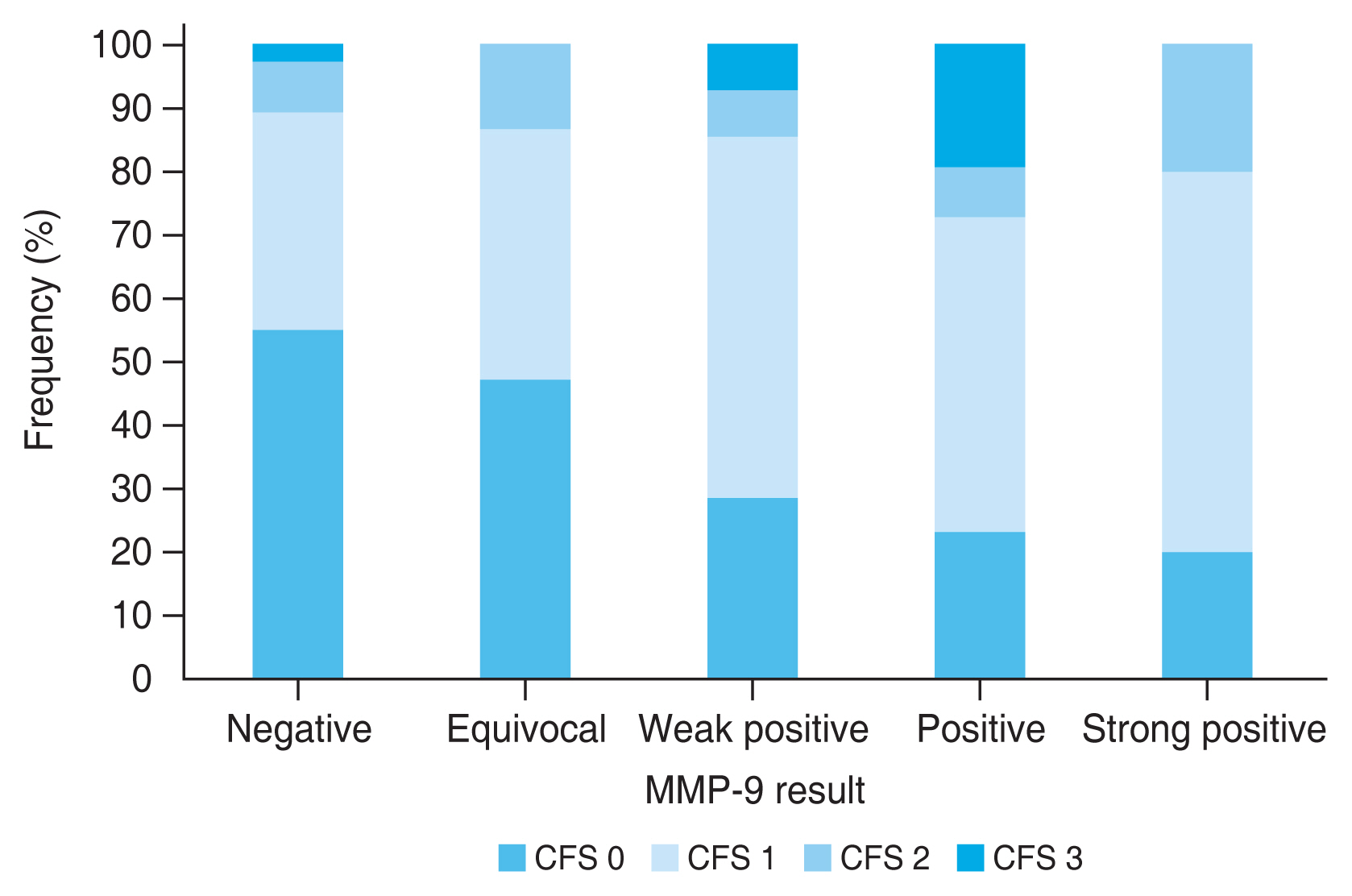
The distribution of corneal and conjunctival fluorescein score (CFS) according to the intensity of positive matrix metalloproteinase-9 (MMP-9) immunoassay results.
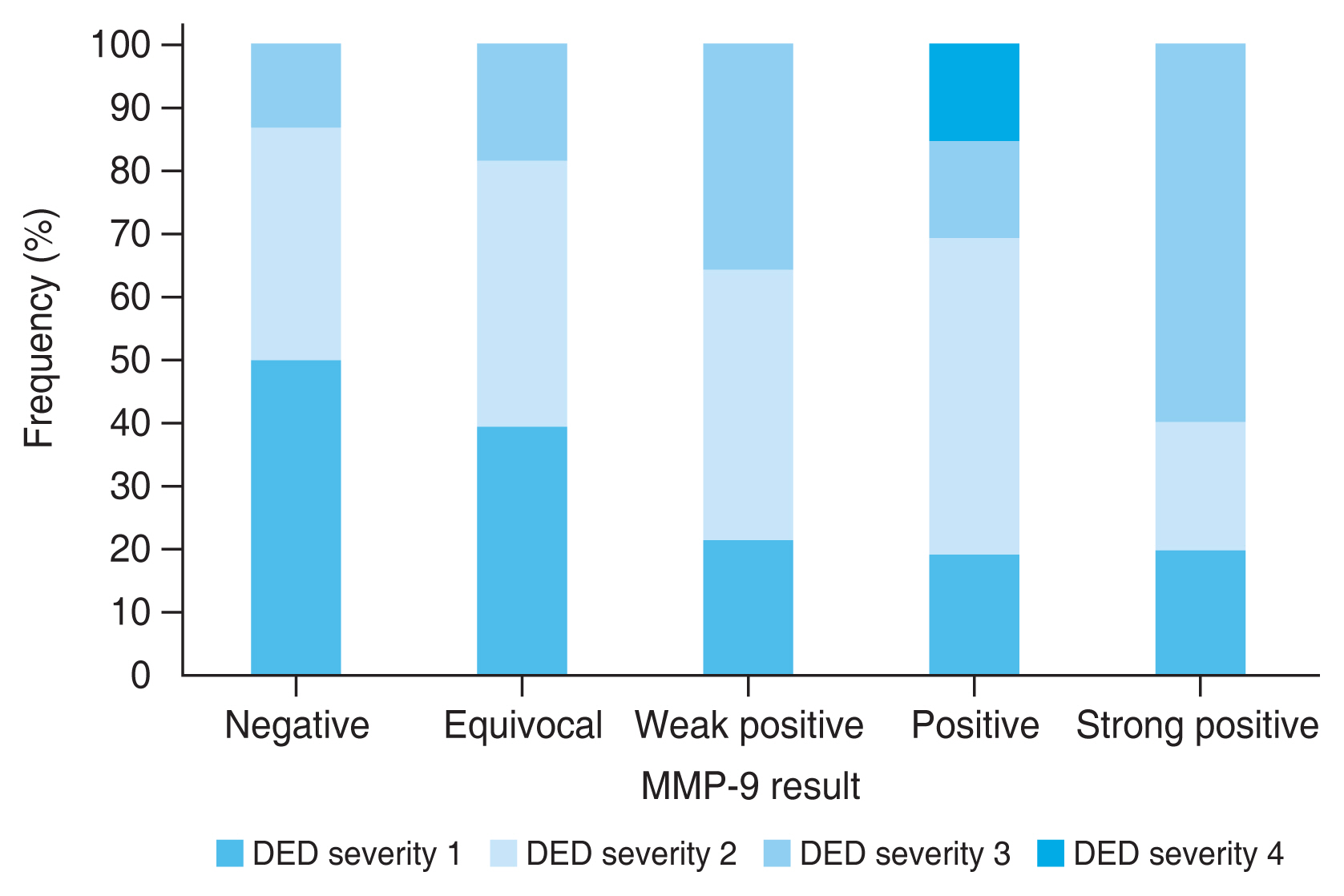
The distribution of dry eye disease (DED) severity grades according to the intensity of positive matrix metalloproteinase-9 (MMP-9) immunoassay results.
The differences in dry eye parameters between those with tear osmolarity of 308 mOsm/L or more and those with tear osmolarity of less than 308 mOsm/L were evaluated (Table 3). There were no significant differences in other dry eye test results, including ocular surface staining score, TBUT, Schirmer I test score, OSDI, MMP-9 positivity, and overall DED severity grade, between the two groups. The differences remained insignificant even when the tear osmolarity cutoff value of 315 mOsm/L was used. The correlation analysis between the tear osmolarity values and other dry eye parameters were also not significant (corneal and conjunctival fluorescein score: r = 0.110, p = 0.227; TBUT: r = −0.022, p = 0.811; Schirmer I test: r = −0.047, p = 0.607; OSDI: r = −0.059, p = 0.520; meibum expressibility: r = −0.169, p = 0.063; meibum quality: r = 0.040, p = 0.659; DED severity: r = 0.016, p = 0.861).

The difference in dry eye parameters between those with higher tear osmolarity of 308 mOsm/L or more and those with less than 308 mOsm/L (n = 125)
In 25 patients with severe DED, the intensity of MMP-9 positivity correlated with ocular surface staining score (r = 0.495, p = 0.016) and DED severity grade (r = 0.475, p = 0.022). The higher tear osmolarity level also showed positive correlation with the ocular surface staining score (r = 0.455, p = 0.029). In 82 ADDE patients, MMP-9 positivity correlated with corneal and conjunctival staining score (r = 0.446, p < 0.001) and TBUT (r = −0.241, p = 0.031). Tear osmolarity level showed positive correlation with DED severity (r = 0.256, p = 0.023). In 53 patients with EDE, MMP-9 positivity correlated with ocular surface staining score (r = 0.342, p = 0.014) and DED severity (r = 0.390, p = 0.005). Tear osmolarity also correlated with corneal and conjunctival staining score (r = 0.392, p = 0.004).
Discussion
MMP-9 positive subjects had higher corneal fluorescein staining score and worse DED severity. The stronger intensity of the test positivity, indicating higher MMP-9 level, was associated with worse ocular surface staining score and DED severity. The correlation between MMP-9 positivity and ocular surface staining remained significant in those with severe DED, ADDE, and EDE. There was no significant difference in dry eye signs and symptoms using the cutoff tear osmolarity level of 308 mOsm/L, however higher tear osmolarity was associated with ocular surface staining score in patients with severe DED and EDE.
The patient’s symptoms often do no correlate with signs of dry eye, and there is no single test that is adequate alone in diagnosing DED. Therefore, a combination of tests such as ocular surface staining, TBUT, and Schirmer test are recommended for diagnosis. Other than these clinical examinations that are subject to variability, more objective tests have become available to aid in the diagnosis of DED. MMP-9 testing and tear osmolarity measurement have been introduced as ancillary diagnostic tests; however, there have been conflicting results on their usefulness.
Tear MMP-9 level was shown to increase proportionally with increasing dry eye severity [6], which was similar to the results of our study. It showed significant correlation with symptom severity scores, decreased low-contrast visual acuity, TBUT, ocular surface fluorescein staining, and topographic surface regularity index, and therefore was suggested as a potential biomarker for diagnosing and monitoring DED [6]. InflammaDry, a rapid point-of-care test for measuring MMP-9, showed sensitivity of 85% and specificity of 94% in diagnosing DED compared to clinical diagnosis using OSDI, Schirmer test, TBUT, and keratoconjunctival staining [10]. It also demonstrated a high positive and negative agreement in diagnosing dry eye against clinical assessment [11]. Another study which used InflammaDry showed that it was positive in 40% of DED patients, and positive results correlated with OSDI, TBUT of less than 5 seconds, Schirmer test, corneal and conjunctival staining, meibomian duct obstruction, and pathologic meibomian gland secretion [12]. In contrast to these favorable results, a previous study did not find significant differences in dry eye symptoms and ocular signs of DED including tear osmolarity, TBUT, corneal staining, Schirmer test, and meibomian gland assessment between MMP-9–positive and MMP-9–negative subjects [13]. Some studies suggested the possibility of false negative results of InflammaDry due to low loading volume [14], and the InflammaDry band density correlated positively with tear meniscus height [15]. The differences in tear volume as well as inadequate collection and transfer of the tear samples could have led to the discrepancies in the results of the previous studies, as well as the inclusion of subjects of different demographics and the difference in the definition of dry eye. However, it was also shown that higher concentration of MMP-9 protein was also associated with higher band density [14], and DED patients with greatest severity (grade 4) showed much higher mean MMP-9 activity (381.24 ± 142.83 ng/mL) compared to control (8.39 ± 4.70 ng/mL) and those who were less severe (grade 1, 35.57 ± 17.04 ng/mL; grade 2, 66.17 ± 57.02 ng/mL; grade 3, 101.42 ± 70.58 ng/mL) [6]. Therefore, the result of our study that InflammaDry band density, thus MMP-9 positivity, increased with dry eye severity coincides with the results of the previous studies. MMP-9 positivity was also associated with increased corneal and conjunctival staining scores, which are indicators of ocular surface damage and inflammation. Therefore, MMP-9 testing can be a valuable tool in identifying the patients with more severe ocular surface involvement and may be used as prognostic indicator for disease severity and progression.
Tear osmolarity, on the other hand, did not show significant association with other dry eye parameters when the cutoff value of either 308 and 315 mOsm/L was used. This suggests that tear osmolarity may not be as informative in assessing ocular surface damage and DED severity as MMP-9 testing. However, previous studies have proved tear osmolarity as a useful tool in diagnosing DED, showing superior diagnostic performance compared to other tests including corneal and conjunctiva staining, TBUT, Schirmer test, and meibomian gland grading [8]. Tear osmolarity correlated with dry eye severity grade (modified Dry Eye Workshop [DEWS] grade) and showed negative correlation with Schirmer test results [16]. Tear osmolarity measurement also showed clinical efficacy in patients with Sjögren syndrome as it was positively correlated with OSDI scores, DEWS classification grades, and corneal and conjunctival staining scores, and negatively correlated with Schirmer test results [17]. A previous study which investigated tear osmolarity and MMP-9 in Sjögren syndrome dry eye showed contradictory results compared to our study [18]. It showed that abnormal tear osmolarity of ≥308 mOsm/L or intereye difference of ≥8 mOsm/L was associated with shorter TBUT, shorter Schirmer test values and higher ocular surface staining score. However, these dry eye indices did not show differences between the MMP-9–positive and MMP-9–negative group. The difference between the study results may be due to the difference in the study population. The previous study included Sjögren syndrome patients, while our study consisted of non-Sjögren dry eye. In fact, tear osmolarity correlated with ocular surface staining score in severe DED patients who may share similar clinical characteristics with those with Sjögren syndrome. Another study which included 757 dry eye patients found that tear osmolarity levels measured with TearLab Osmolarity System did not differ between the patient group and the control group regardless of cutoff value (>308 and >316 mOsm/L and intereye difference >8 mOsm/L) [19]. Moreover, the tear osmolarity cutoff values could not discriminate DED pathological scores including OSDI, TBUT, ocular surface staining, and meibomian gland functionality. These results are similar to our study which did not show significant results using similar cutoff values of tear osmolarity. Similarly, a study found that there was no association between tear osmolarity values and symptoms and other clinical parameters of dry eye including TBUT, fluorescein staining and Schirmer test [20]. Our study found that although the cutoff values of 308 and 315 mOsm/L was not significant, the higher tear osmolarity correlated positively with ocular surface staining score in severe DED and EDE patients. It also correlated with DED severity in ADDE patients. Therefore, tear osmolarity may be useful in those with clinically significant DED in determining disease severity. The disparities in the study results could be due to the variability in tear osmolarity measurement and different clinical characteristics of the study subjects and dry eye definition. Tear osmolarity measurement are subject to variability and can be affected by various environmental factors such as temperature and humidity. It is also subject to measurement error and may differ according to the examiner technique and the type of osmometers. The unstable nature of tear film in DED may also hinder accurate measurement of tear osmolarity. Standardization of tear osmolarity measurement protocols and further investigation may be needed to clarify its role in DED diagnosis and monitoring.
An in vitro study found that concentrations often measured on the ocular surface (312 mOsm/L) was not enough to initiate inflammatory response and a higher osmolarity concentration (450–500 mOsm/L) was needed to induce MMPs including MMP-9 that disrupt the epithelial barrier and trigger a perpetuating inflammation, thus aggravating dry eye severity [21]. Although most in vitro studies use hyperosmolarity of higher levels (400–600 mOsm/L) than in vivo studies, the tear osmolarity level of our study subjects (317.62 ± 24.44 mOsm/L) may not have been high enough to show significant increase in inflammatory markers including MMP-9, and therefore it could be the reason for the lack of correlation between the tear osmolarity level and MMP-9 positivity in our study.
The limitations of this study include a relatively small sample size and inclusion of patients with diverse ocular surface findings. The clinical measurements were collected at one point in time, and therefore the variability could not be assessed or adjusted. Future longitudinal studies would allow for better understanding of disease progression and the predictive values of biomarkers such as MMP-9.
Our study showed that MMP-9 positivity was significantly associated with higher corneal and conjunctival fluorescein scores and worse DED severity, however the suggested cutoff tear osmolarity values of 308 and 315 mOsm/L could not differentiate severity. These findings highlight the importance of utilizing a combination of tests for the assessment of DED, as no single test alone can adequately capture the complexity of the disease. MMP-9 testing, in conjunction with other clinical examinations, may aid in identifying patients with more severe ocular surface involvement.
In conclusion, MMP-9 positivity was associated with higher ocular surface staining score and worse dry eye severity. DED is a complex, heterogenous condition in which multiple diagnostic tests and clinical examinations are needed for diagnosis and assessment to guide in targeted therapeutic intervention and disease monitoring.
Acknowledgements
None.
Notes
Conflicts of Interest: None.
Funding: This work was supported by a National Health Insurance Service Ilsan Hospital grant (No. NHIMC-2022-CR-027-2022-CR-027).