Short-term Efficacy and Safety of Intravitreal Brolucizumab Injection for Treatment-Naive Exudate Age-related Macular Degeneration: A Multicenter Study
Article information
Abstract
Purpose
To compare short-term efficacy and safety of intravitreal brolucizumab injection with aflibercept in treatment-naive neovascular age-related macular degeneration (nAMD) patients.
Methods
A total of 59 eyes from 59 treatment-naive nAMD patients in three hospitals were retrospectively reviewed. Of which, 27 patients underwent intravitreal brolucizumab injections and 32 received aflibercept. After monthly consecutive three injections, best-corrected visual acuity (BCVA; in logarithm of minimal angle of resolution [logMAR]), central macular thickness (CMT), dry macula achievement rate, and intraocular inflammation (IOI) incidence were compared.
Results
After loading-phase treatment, BCVA was significantly increased from 0.48 ± 0.30 logMAR at baseline to 0.33 ± 0.21 logMAR at 3 months in the brolucizumab group (p = 0.002) and 0.40 ± 0.39 logMAR at baseline to 0.33 ± 0.36 logMAR at 3 months in the aflibercept group (p = 0.007). But there was no significant difference in BCVA improvement at 3 months between the two groups. CMT significantly decreased from 429.67 ± 250.59 μm at baseline to 210.67 ± 93.53 μm at 3 months in the brolucizumab group and from 346.69 ± 159.09 μm to 234.52 ± 83.42 μm in the aflibercept group (both p < 0.001). The amount of CMT reduction was significantly greater in the brolucizumab group after 3 months (p = 0.036). In typical AMD eyes, brolucizumab showed similar BCVA improvement but better CMT reduction at 3 months (p = 0.018). Dry macula achievement rate was not significantly different between the two groups. One IOI was observed in the brolucizumab group.
Conclusions
Intravitreal injections of brolucizumab and aflibercept showed similar anatomical and functional outcomes. But CMT reduction was greater in the brolucizumab group. One IOI was identified, which was tolerable for topical agents. These results suggest that brolucizumab could be a novel first line treatment option for treating naive nAMD patients.
Age-related macular degeneration (AMD) is one of the leading causes of severe vision loss in advanced countries. It can be divided into non-neovascular AMD and neovascular AMD (nAMD) [1–4]. Growth of choroidal neovascularization (CNV) in nAMD is the main cause of vision loss [5,6]. Laser photocoagulation, photodynamic therapy, and intravitreal anti–vascular endothelial growth factor (anti-VEGF) injection are widely used treatments for nAMD. Since the introduction of intravitreal anti-VEGF injection, the number of patients who suffer severe vision loss due to nAMD has profoundly reduced [7–9]. Several anti-VEGF agents including bevacizumab, ranibizumab, and aflibercept are used globally [10]. Brolucizumab (Beovu, Novartis AG) is a single chain antibody fragment with 26 kDa molecular mass that can inhibit all isoforms of VEGF-A. It was approved by the US Food and Drug Administration in 2019 [11–13]. Compared to other anti-VEGF agents, it has smaller molecular weight and higher solubility. Therefore, it can be administered in high concentrations with stronger anti-VEGF effect than other anti-VEGF agents. Previous phase III studies (HAWK and HARRIER trials) have shown that every 12 weeks and every 8 weeks dosing intervals of intravitreal brolucizumab injection are not inferior to every 8 weeks dosing intervals of intravitreal aflibercept injection in best-corrected visual acuity (BCVA) improvement and superior in intraretinal fluid (IRF), subretinal fluid (SRF), and subretinal pigment epithelium (sub-RPE) fluid reductions [14,15]. Although brolucizumab recently started to be used widely around the world, HAWK and HARRIER studies reported that 4.6% of studied eyes showed intraocular inflammation (IOI) [14,15]. Numerous IOI cases have been reported after intravitreal brolucizumab injections [16,17]. But in the country, there is no study compared brolucizumab to other anti-VEGF agents so far. Thus, this study aimed to evaluate short-term effect of intravitreal brolucizumab injection compared to aflibercept, which is widely used in this country for treating naive nAMD patients. Incidence of adverse events of brolucizumab was also checked.
Materials and Methods
The study protocol adhered to the tenets of the Declaration of Helsinki. The study was approved by the Institutional Review Board of Soonchunhyang University Hospital (No. 2022-07-009). The requirement for informed consent was waived due to the retrospective nature of the study.
In this multicenter retrospective study, medical records of treatment-naive nAMD patients administered with intravitreal brolucizumab or aflibercept injections as the first line therapy monthly with three consecutive injections as a loading phase in three Soonchunhyang University Hospitals (Seoul, Bucheon, and Cheonan, Korea) during the period of November 2021 to August 2022 were reviewed.
Patients who had a previous history of vitreoretinal surgery or glaucoma were excluded. The presence of choroidal neovascularization was defined as a leakage in indocyanine green angiography (ICGA). When polyp-like choroidal vessel dilatation was observed in ICGA, a diagnosis of polypoidal choroidal vasculopathy (PCV) was made. At baseline, all subjects underwent comprehensive ophthalmic examinations including BCVA, intraocular pressure (IOP), slit-lamp examinations, fundus examinations, fundus photography, optical coherence tomography (OCT), fundus fluorescein angiography, and ICGA. At 1 month and 3 months after the first injection, BCVA, IOP, slit-lamp examinations, fundus examinations, fundus photography, and OCT were performed for all subjects. We also reviewed their age, sex, and past medical history (Fig. 1). For OCT images, spectral-domain OCT (SD-OCT; Spectralis OCT, Heidelberg Engineering) and SD-OCT with AngioVue Imaging System (RTVue XR 100 Avanti, Optovue Inc) were used. Central macular thickness (CMT) was defined as the distance between the internal limiting membrane and the RPE surface at the fovea on a horizontal OCT scan image. After a loading-phase treatment, we analyzed OCT images and assessed dry macula achievement which meant absence of SRF or IRF on the OCT images.
For statistical analyses, BCVA was converted into logarithm of minimal angle of resolution (logMAR) units. Chisquare independence test was used to compare dry macula achievement rate, IOI incidence, and baseline demographics between the two groups. The Wilcoxon signed-rank test was used for intergroup comparisons of BCVA, IOP, and CMT after injections. The Mann-Whitney U-test was used to compare differences in BCVA, IOP, CMT, and baseline demographics between the two groups. All statistical analyses were performed using IBM SPSS ver. 21.0 (IBM Corp). A p < 0.05 was considered statistically significant.
Results
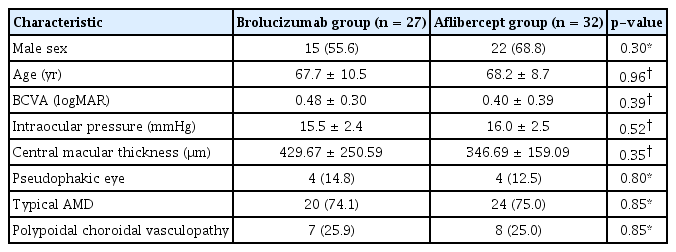
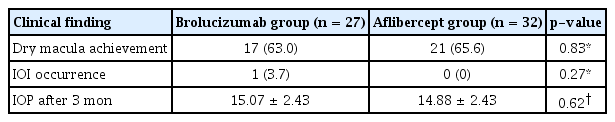
A total of 59 eyes from 59 patients were enrolled in this study. Of which, 27 eyes underwent intravitreal brolucizumab injections and 32 eyes received intravitreal aflibercept injections. The mean age of patients was 67.7 ± 10.5 years in the brolucizumab group and 68.2 ± 8.7 years in the aflibercept group. At baseline, demographic and clinical characteristics of patients showed no significant difference between the two groups (Table 1). BCVA at baseline, 1 month, and 3 months after treatment were 0.48 ± 0.30, 0.38 ± 0.25, and 0.33 ± 0.21 logMAR, respectively, in the brolucizumab group; and 0.40 ± 0.39, 0.35 ± 0.33, and 0.33 ± 0.36 logMAR, respectively, in the aflibercept group. Compared to baseline BCVA, both groups showed significant improvement at 1 month (brolucizumab group, p = 0.012; aflibercept group, p = 0.025) and 3 months (brolucizumab group, p = 0.002; aflibercept group, p = 0.007) after treatment. However, BCVA showed no significant difference between the two groups at 1 month (p = 0.327) or 3 months (p = 0.335) after treatment (Fig. 2). In typical AMD eyes, BCVA at baseline, 1 month, and 3 months after treatment were 0.42 ± 0.30, 0.34 ± 0.27, and 0.32 ± 0.23 logMAR, respectively, in the brolucizumab group; and 0.44 ± 0.42, 0.38 ± 0.36, and 0.36 ± 0.38 logMAR, respectively, in the aflibercept group. At baseline, BCVA showed no significant difference between the two groups (p = 0.749). Compared to baseline BCVA, BCVA at 3 months after treatment was significantly improved in both groups (brolucizumab group, p = 0.021; aflibercept group, p = 0.007). However, BCVA improvement was not statistically significant after 1 month of treatment in either group (brolucizumab group, p = 0.082; aflibercept group, p = 0.119). There was no significant difference in BCVA between the two groups at 1 month (p = 0.943) or 3 months (p = 0.915) after treatment (Fig. 3). In PCV eyes, BCVA at baseline, 1 month, and 3 months after treatment were 0.66 ± 0.23, 0.49 ± 0.15, and 0.37 ± 0.12 logMAR, respectively, in the brolucizumab group; and 0.32 ± 0.26, 0.26 ± 0.22, and 0.24 ± 0.26 logMAR, respectively, in the aflibercept group. At baseline, BCVA showed significant difference between the two groups (p = 0.040). Compared to baseline BCVA, BCVA improvement was significant in both groups at both 1 month (brolucizumab group, p = 0.039) and 3 months (brolucizumab group, p = 0.042; aflibercept group, p = 0.046) except for BCVA at 1 month in the aflibercept group ( p = 0.104) (Fig. 4). CMT values at baseline, 1 month, and 3 months were 429.67 ± 250.59, 231.85 ± 112.38, and 210.67 ± 93.53 μm, respectively, in the brolucizumab group and 346.69 ± 159.09, 273.22 ± 92.45, and 234.52±83.42 μm, respectively, in the aflibercept group, showing significant reductions at both 1 month (both p < 0.001) and 3 months (both p < 0.001). CMT reduction was significantly greater in the brolucizumab group than in the aflibercept group at both 1 month (p = 0.005) and 3 months (p = 0.036) (Fig. 5). In typical AMD eyes, CMT at baseline, 1 month, and 3 months were 364.40 ± 179.60, 212.50 ± 73.67, and 193.65±67.85 μm, respectively, in the brolucizumab group; and 367.39 ± 175.50, 272.13 ± 99.84, and 233.96 ± 86.90 μm, respectively, in the aflibercept group. At baseline, CMT showed no significant difference between the two groups (p = 0.888). Compared to baseline CMT, CMT reduction was significant in both groups at both timepoints (all p < 0.001). CMT reduction was significant in the brolucizumab group at both timepoints (at 1 month, p = 0.005; at 3 months, p = 0.018) (Fig. 6). In PCV eyes, CMT at baseline, 1 month, and 3 months were 616.14 ± 321.10, 287.14 ± 170.54, and 259.29 ± 131.90 μm, respectively, in the brolucizumab group and 293.78 ± 85.96, 276.00 ± 70.03, and 254.89 ± 74.63 μm, respectively, in the aflibercept group. At baseline, CMT showed no significant difference between the two groups (p = 0.072). Compared to baseline CMT, CMT reduction was significant in both groups at both timepoints (brolucizumab group at 1 month and 3 months, p = 0.018; aflibercept group at 3 months, p = 0.012) except for CMT at 1 month in the aflibercept group (p = 0.123). There was no significant difference between the two groups (at 1 month, p = 0.232; at 3 months, p = 0.336) (Fig. 7). Dry macula was achieved in 17 eyes out of 27 eyes in the brolucizumab group and 21 eye out of 32 eyes in the aflibercept group, showing no significant difference between the two groups ( p = 0.83) (Table 2). During the follow-up period, IOI occurred in only one eye (typical nAMD) in the brolucizumab group, showing no significant difference between the two groups (p = 0.27) (Table 2). Compared to IOP at baseline, IOP at 3 months was not significantly different in either group (brolucizumab group, p = 0.122; aflibercept group, p = 0.125). Furthermore, IOPs after 3 months were not significantly different between the two groups (p = 0.62) (Table 2).
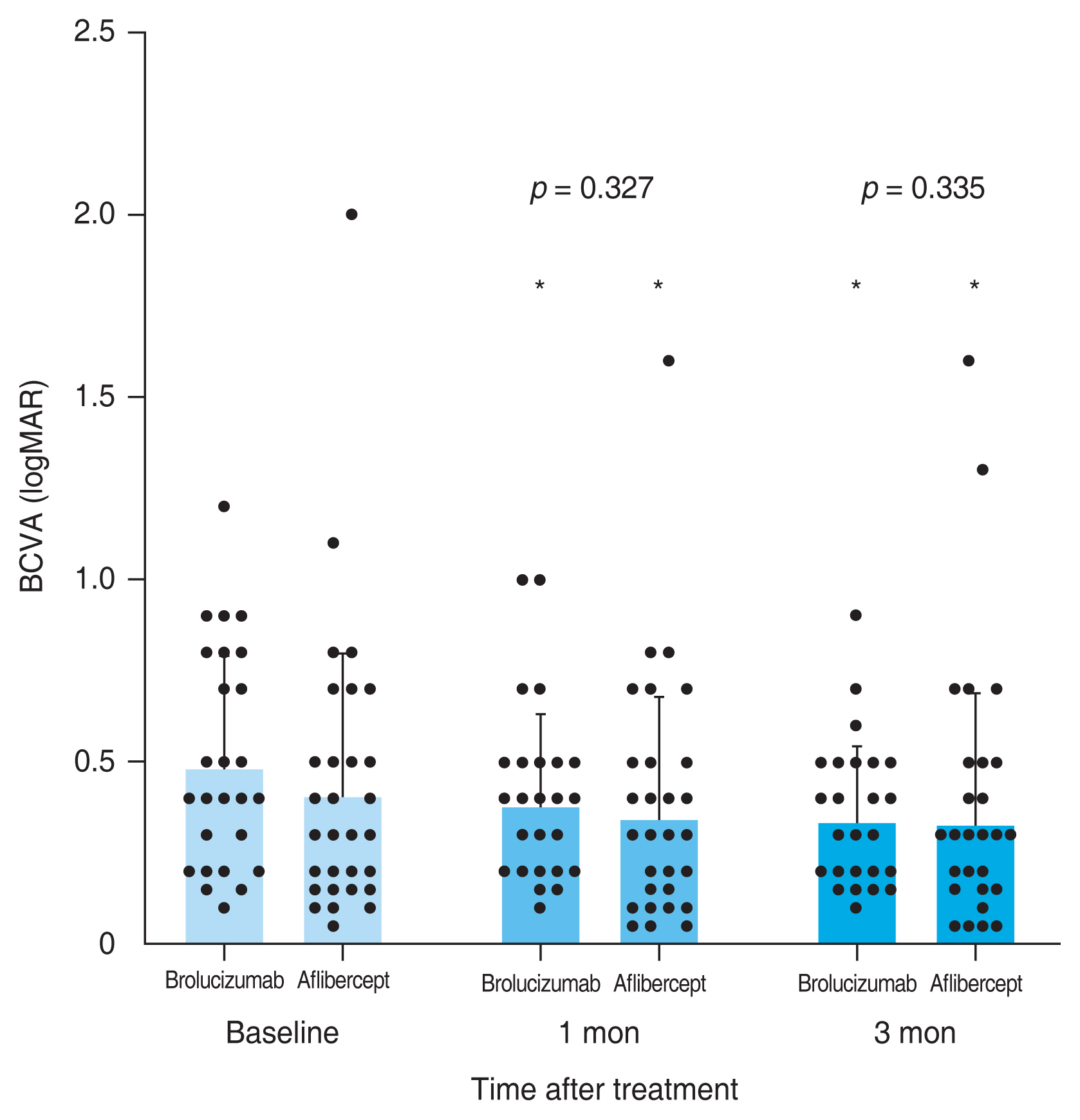
Consecutive changes of the mean best-corrected visual acuity (BCVA). Mean BCVA was significantly improved after 1 and 3 months in both groups (*p < 0.05). However, there was no significant difference between the two groups (at 1 month, p = 0.327; at 3 months, p = 0.335). Measurements are presented as dots. logMAR = logarithm of minimal angle of resolution.
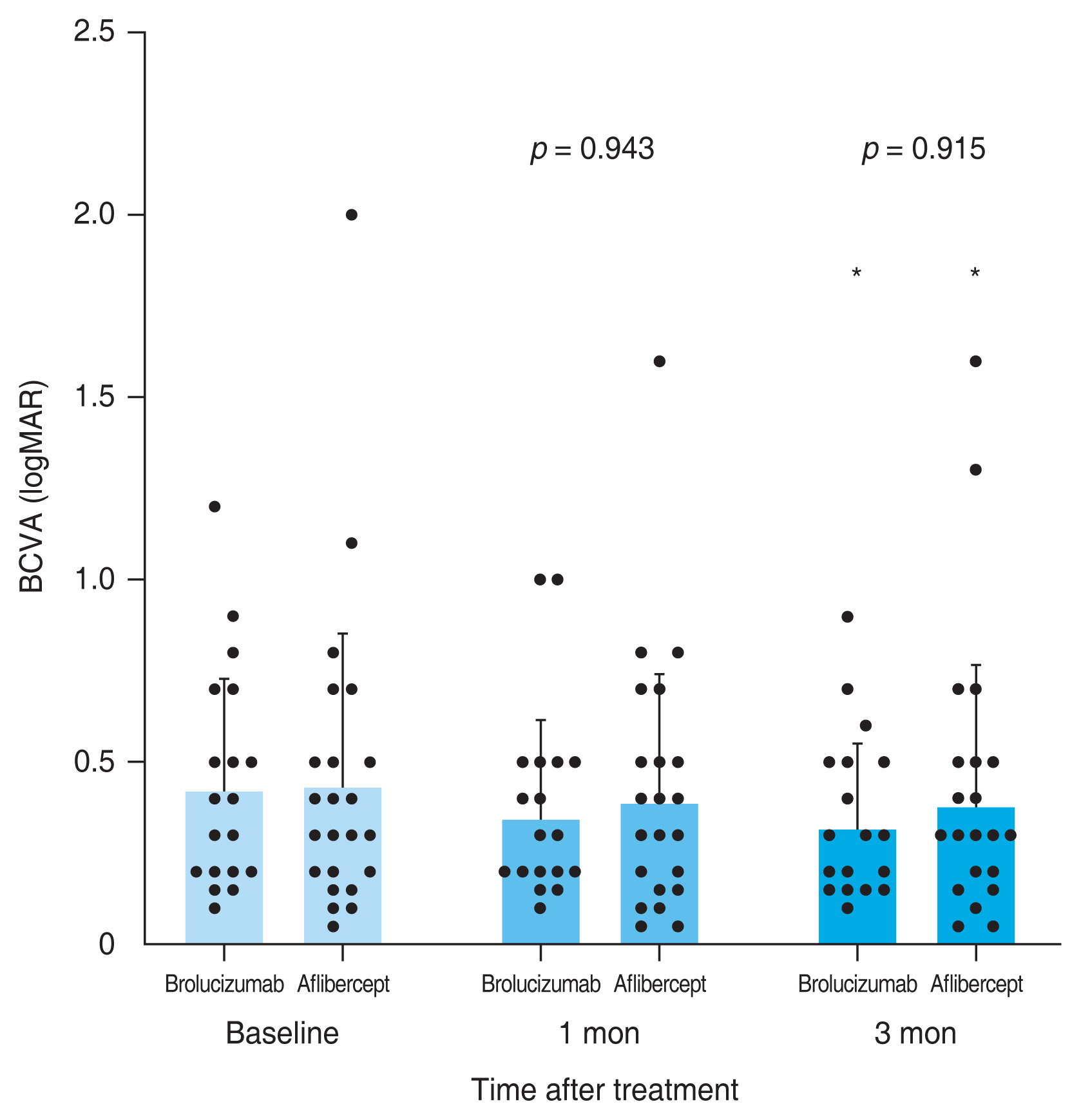
Consecutive changes of the mean best-corrected visual acuity (BCVA) in typical age-related macular degeneration eyes. Mean BCVA was significantly improved after 3 months in both groups (*p < 0.05). However, there was no significant difference between the two groups (at 1 month, p = 0.943; at 3 months, p = 0.915). Measurements are presented as dots. logMAR = logarithm of minimal angle of resolution.
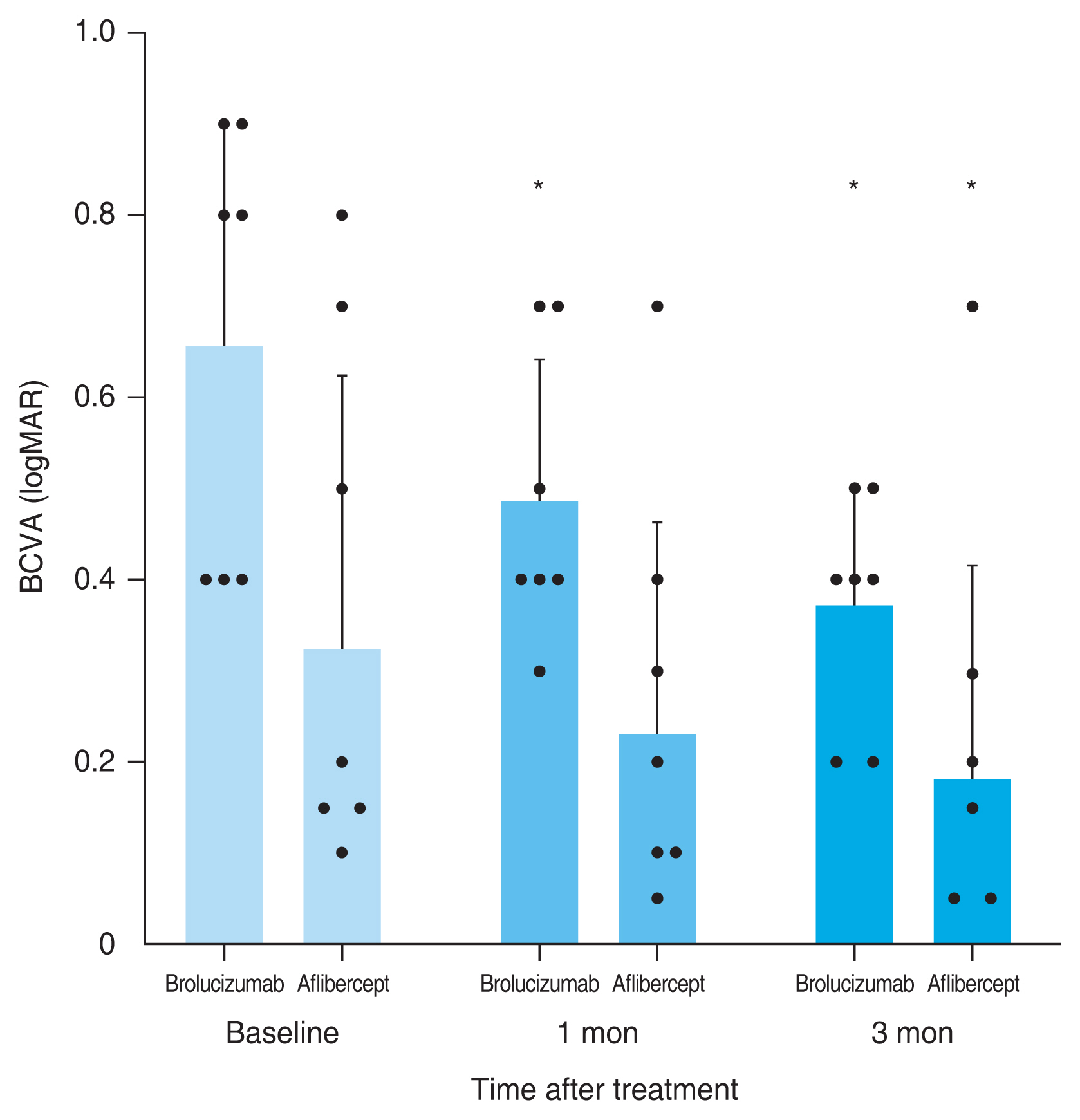
Consecutive changes of the mean best-corrected visual acuity (BCVA) in polypoidal choroidal vasculopathy eyes. Mean BCVA was significantly improved after 1 month in the brolucizumab group and 3 months in both groups (*p < 0.05). Measurements are presented as dots. logMAR = logarithm of minimal angle of resolution.
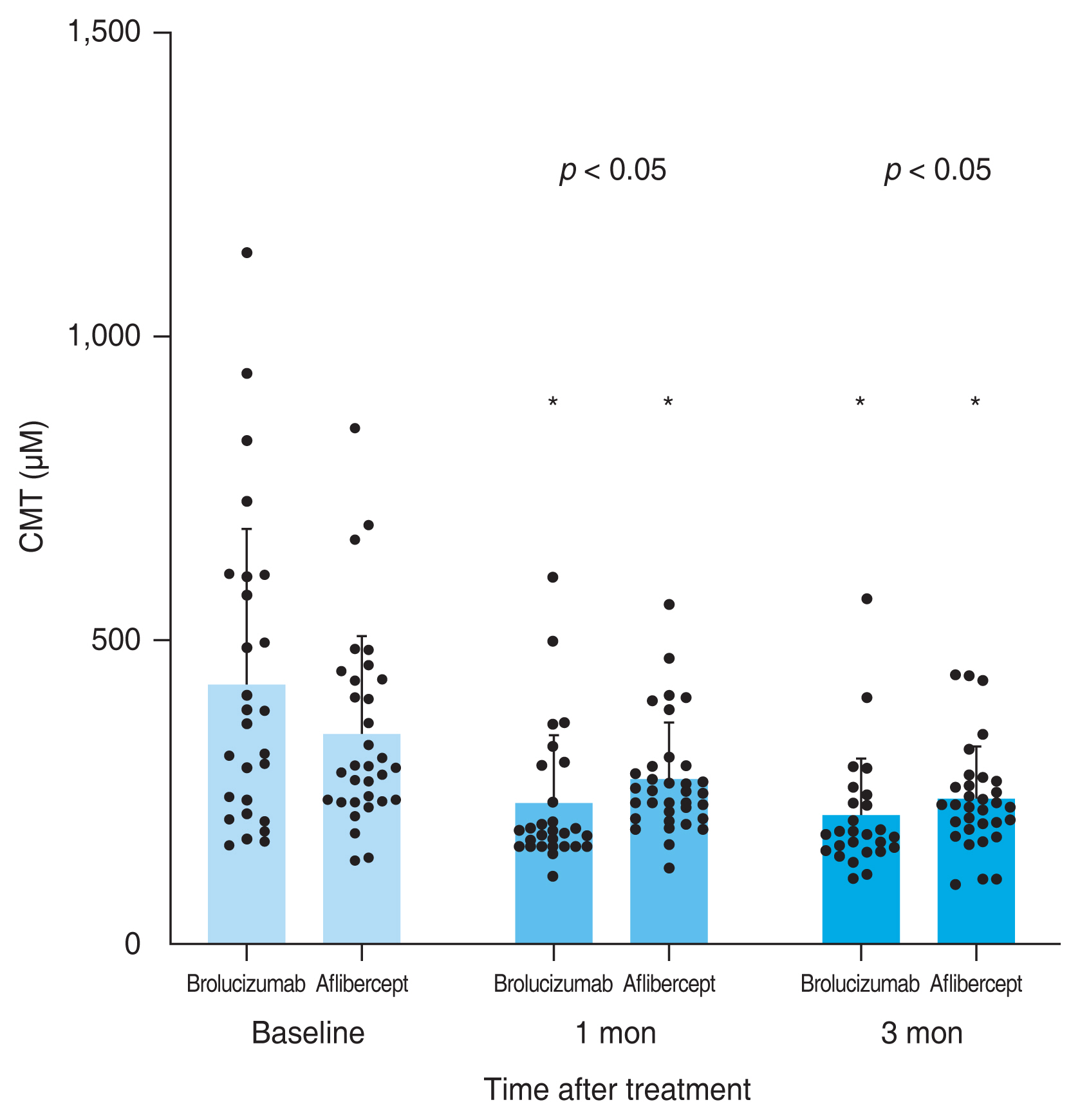
Consecutive changes of the mean central macular thickness (CMT). Mean CMT was significantly reduced after 1 and 3 months in both groups (*p < 0.05). The reduction was significantly greater in the brolucizumab group compared to the aflibercept group after 1 and 3 months (p < 0.05). Measurements are presented as dots.
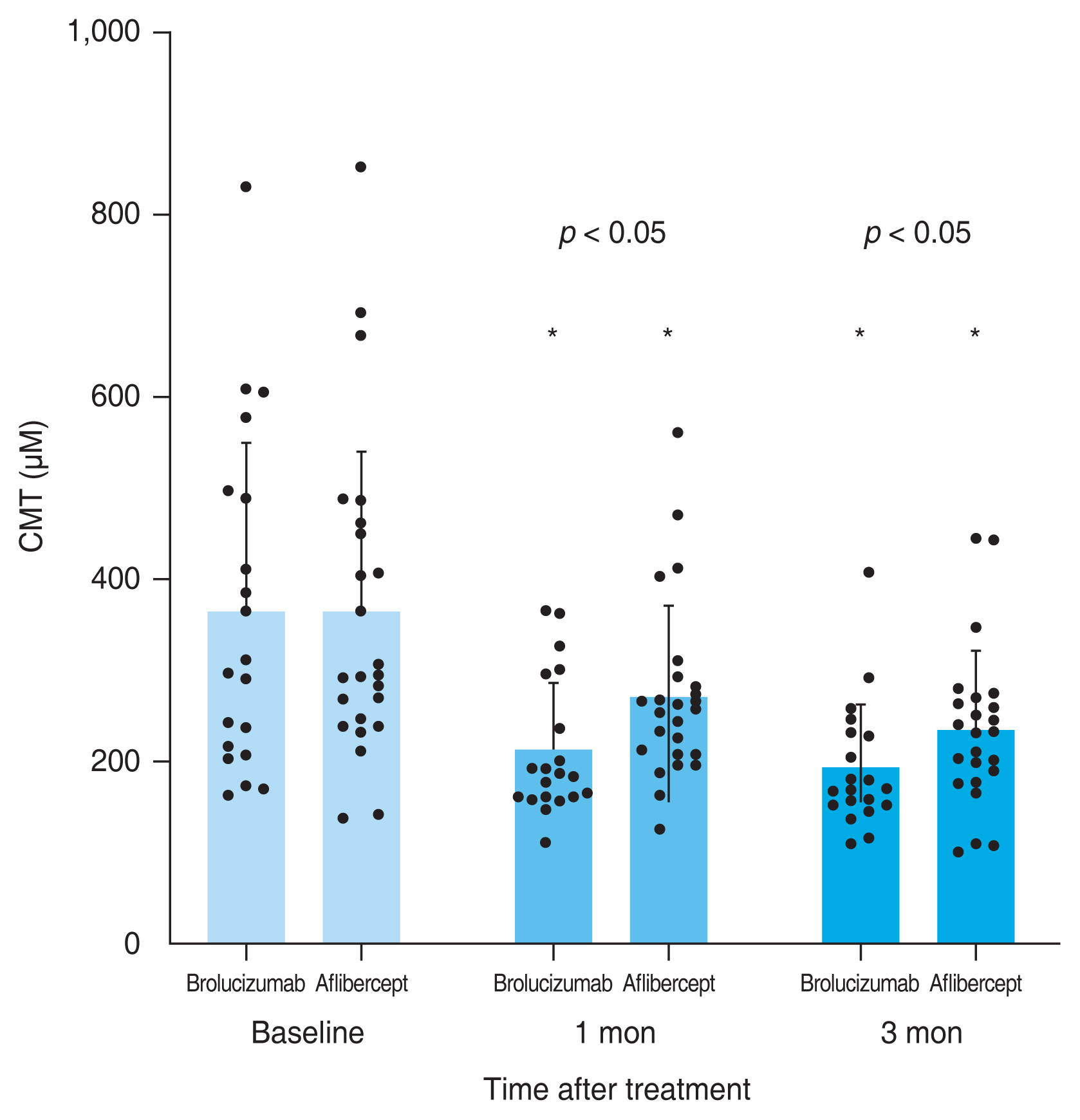
Consecutive changes of the mean central macular thickness (CMT) in typical age-related macular degeneration eyes. Mean CMT was significantly reduced after 1 and 3 months in both groups (*p < 0.05). The reduction was significantly greater in the brolucizumab group compared to the aflibercept group after 1 and 3 months (p < 0.05). Measurements are presented as dots.
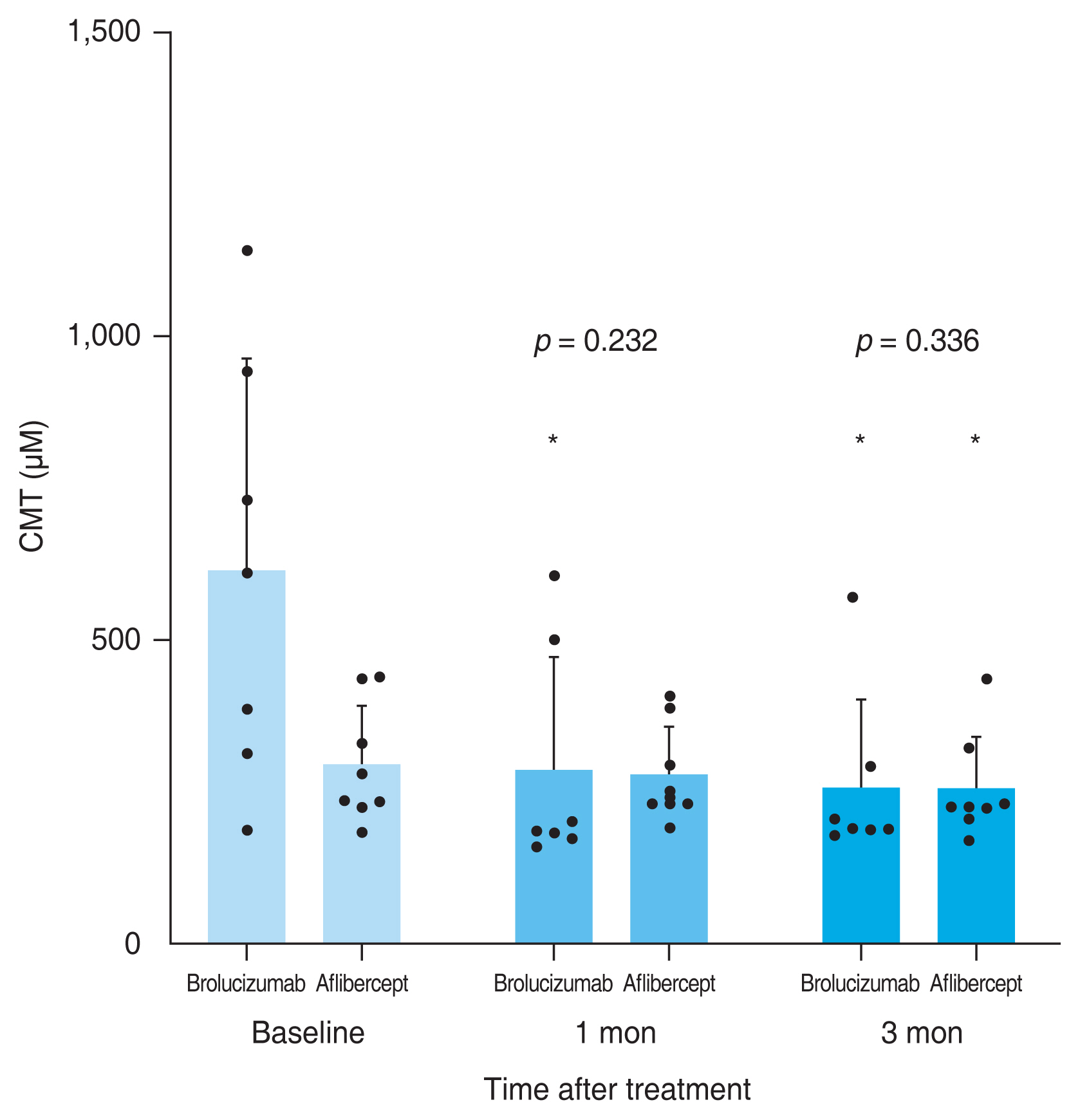
Consecutive changes of the mean central macular thickness (CMT) in polypoidal choroidal vasculopathy eyes. Mean CMT was significantly reduced after 1 month in the brolucizumab group and 3 months in both groups (*p < 0.05). However, there was no significant difference between the two groups (at 1 month, p = 0.232; at 3 months, p = 0.336). Measurements are presented as dots.
Discussion
This retrospective study compared clinical efficacy and adverse event following intravitreal brolucizumab injection compared to aflibercept in treatment-naive nAMD patients during loading-phase treatment. In previous studies, brolucizumab resulted in similar BCVA improvement and CMT reduction but greater central choroidal thickness reduction than aflibercept [14,15,18]. In the present study, BCVA was significantly improved and CMT was significantly decreased in both groups. Although there was no significant difference in BCVA improvement between the two groups, CMT reduction was significantly higher in the brolucizumab group at both 1 month and 3 months after the first injection. We then divided subjects into typical AMD and PCV. In typical AMD patients, BCVA was significantly improved in both groups at 3 months. CMT reduction was significant in both groups at both 1 month and 3 months. Between the two groups, BCVA improvement was not significantly different, although CMT reduction was significantly higher in the brolucizumab group at 1 month and 3 months, showing similar results in total eyes. In PCV patients, BCVA was significantly improved at 1 month in the brolucizumab group and at 3 months in both groups. CMT reduction was significant at 1 month in the brolucizumab group and at 3 months in both groups. However, there was a significant difference in BCVA at baseline between the two groups. Due to the small number of participants, authors could not compare the effect of brolucizumab in BCVA improvement to aflibercept. For CMT reduction, brolucizumab showed comparable CMT reduction compared to aflibercept. In previous studies, brolucizuamb showed no significant difference in BCVA improvement or CMT reduction compared to aflibercept in PCV eyes [19,20]. These differences might be due to a small number of participants.
In HAWK and HARRIER studies, brolucizumab showed better resolution of SRF, IRF, and sub-RPE fluid than aflibercept [14,15]. Other studies have shown that brolucizumab is superior in fluid reduction in nAMD patients, including refractory nAMD patients [14,21–23]. In the present study, 63% of patients in the brolucizumab group achieved dry macula, although dry macula achievement rate was not significantly different between the two groups after loading-phase treatment. Such discrepancy with previous studies might be due to the small number of subjects enrolled in the present study and a short-term study period.
It has been generally considered that IOI is the most frightening complication of intravitreal brolucizumab injection. Previous studies have reported that brolucizumab can induce IOI more frequently than other anti-VEGF agents [14–17,24–29]. It is mostly controlled with topical or systemic steroid administration. In some cases, however, brolucizumab caused retinal vasculitis and vascular occlusion that could induce severe vision loss [24–28]. The cause of IOI has not fully understood yet. Reported risk factors of brolucizumab injection include old age, female sex, and history of diabetes. There are no specific ways to predict or prevent brolucizumab induce IOI. Therefore, the most important thing is to educate patients and detect and treat IOI with steroid as soon as possible [24,25,29]. In this study, one eye in brolucizumab group experienced IOI after the third injection. It resolved completely with topical steroid. Incidence of IOI showed no significant difference between the two groups.
Limitations of this study include its retrospective design and a relatively small number of eyes despite the fact that it is a multicenter study. Especially for PCV patients, results were inaccurate and hard to analyze because the number of patients were too small. Further studies with more PCV patients are necessary to compare the effect of brolucizumab with that of aflibercept. In addition, we only studied loading-phase treatment period (from baseline to 3 months), meaning that we did not have long term data. Thus, further investigation is needed. These factors might have contributed to different results in dry macula achievement rate and IOI incidence compared to previous studies.
In conclusion, loading-phase treatment with intravitreal brolucizumab for treatment-naive nAMD patients is equally effective in BCVA improvement and dry macula achievement. In addition, brolucizumab resulted in better CMT reduction at 3 months. Therefore, brolucizuamb can be a good first line treatment option for treatment-naive nAMD patients, although close observation for IOI occurrence is needed.
Acknowledgements
None.
Notes
Conflicts of Interest: None.
Funding: This report was supported by the Soonchunhyang University Research Fund. The funders had no role in case selection, decision to publish, or preparation of the manuscript.