Clinical Implication of Dacryoendoscopy in the Patients with Tearing: A Systematic Review
Article information
Abstract
Purpose
A systematic review of the literature on diagnostic and therapeutic indications, techniques, and complications of dacryoendoscopy (DE) was performed.
Methods
The authors performed a PubMed search of articles published in English on DE. Data were collected and classified according to the categories of the disease. The clinical outcomes and limitations were particularly analyzed.
Results
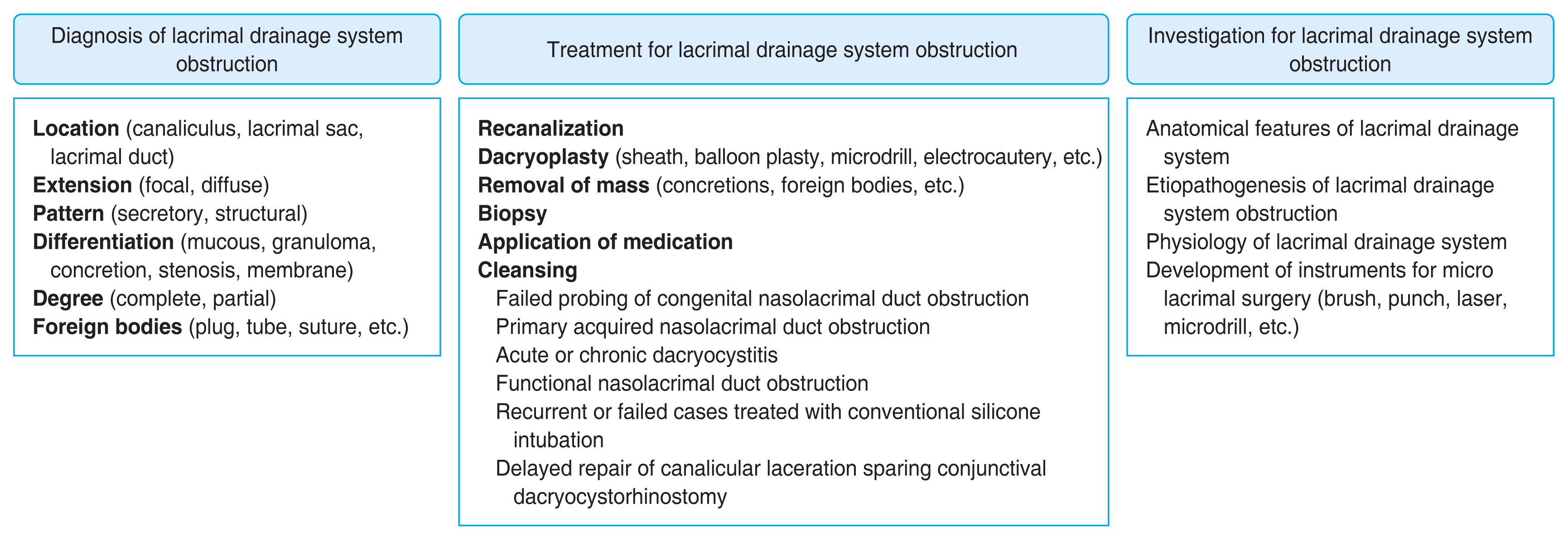
The lacrimal drainage system from the canaliculus to the inferior meatus could be examined based on the specific anatomical features by DE. The canalicular mucosa is smooth and brightly colored, the lacrimal sac shows covering mucosa with good vascularization and the nasolacrimal duct is lined with bright tubular mucosal folds. DE allows direct visualization of the detailed internal condition of the lacrimal disorders, to directly diagnose the site of obstruction with accuracy and address the causes and recanalize the lacrimal drainage system using assisted micro lacrimal surgical instruments in the tearing patients.
Conclusions
Better visualization of the lacrimal canal with DE improves the understanding of physiology and precise identification of the obstructing lesions, both of which are the key to a comprehensive management for the tearing patients.
Introduction
Modern lacrimal surgical techniques provide a large spectrum of therapeutic modalities enabling patient care to be minimally invasive and customized using dacryoendoscopy (DE) since 2000. DE is the microendoscopy of lacrimal drainage system (LDS), which visualize the canaliculi, common canaliculus, lacrimal sac, and nasolacrimal duct (NLD). DE facilitates us to examine the detailed morphologically inside the LDS and its disorders [1].
A semirigid modular endoscope or vitroptik (Modular Salivascope, PolyDiagnost) with field of view 70°, viewing direction 0°, 6,000 pixels, outer diameter 0.53 mm, an eye-piece connection for a video camera and with two or three working channels have reproduced good quality images [2–4]. They also developed a flexible fiber optic ranging from an outer diameter of 0.75 to 1.10 mm (PolyDiagnost) with a 6,000-pixel image resolution inside LDS. Furthermore, polypoid mucosa or strictures can be treated with a laser, and obstructions can be widened mechanically and space occupying lesions like dacryoliths or foreign bodies could be also removed by a microdrill [5]. The dacryoendoscope manufactured by FiberTech (RUIDO fiberscope MD10) included conventional and high-definition ones. RUIDO fiberscope MD10 (available in 0.7 and 0.9 mm) is a rigid endoscope consisting of a viewing lens, 10,000-pixel fiber optic cable, and irrigation channel angled 27° at 10 mm from the tip [1]. It also provides high-definition DE (HDD) with 15,000 pixel fiber optic cable and 60° field view [1]. Microendoscope by Karl Storz (Miniature Telescope 0 Degree, 110° field of view with 0.65–0.85 mm diameter) using miniaturized optic fiber with three working ports is also available, which gives better image quality (Fig. 1A–1F) [1]. Microendoscopy assisted instruments such as laser or microdrill has been variously applied and reported to be successful in treating LDS disorders. This review collected the various techniques, indications, and clinical outcomes of DE in the tearing patients.
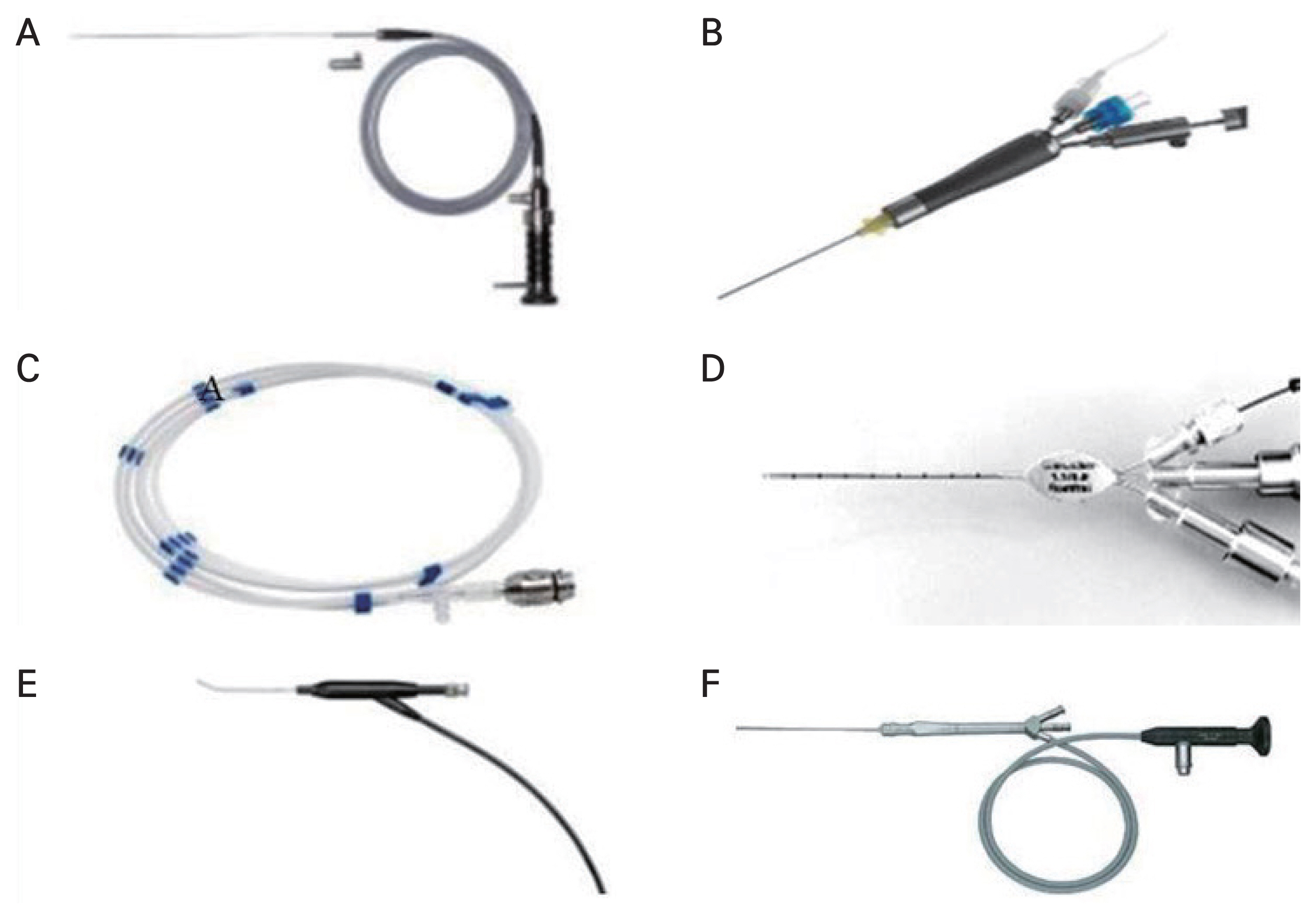
Various types of dacryoendoscopy. (A,B) Semirigid endoscope (Modular Salivascope, PolyDiagnost) was applied with endoscopic blunt cannula as a probe with lumen in the past, but it is combined with polyshaft and handle with three ports for working channel and irrigation channel. (C,D) Flexible endoscopy system (PolyDiagnost) consists of the flexible optic scope and modified probe or modular catheter with working channels. (E) Rigid RUIDO fiberscope (MD10, Fibertech) was introduced as a bent shaped dacryoendoscope. (F) Microendoscope (Miniature Telescope 0 Degree, Karl Storz) is used as a shaft containing miniaturized fiber optics, irrigation and interventional channel.
Methods
A systematic MEDLINE search was performed on PubMed using the terms “dacryoendoscope,” AND “dacryoendoscopy.” There was a restriction on the date of publication before in 2000. Articles published in English on DE, and relevant cross-references were included for the review. References include instrumentation, techniques, diagnostic and therapeutic indications, outcomes, and complications. Specific emphasis was laid on addressing the current practice patterns, clinical applications and future direction.
Results
Assisted techniques of DE
Regarding DE, three-port system with an external diameter of 1.1 mm and internal diameter of 0.7 to 0.9 mm can permit the additional simultaneous use of microdrills, laser probes, micropunches, and miniature balloons [6]. Microdrill dacryoplasty utilizes battery powered miniaturized motor drills of 0.3 mm diameter, which can be easily incorporated along with the DE [7]. Wang et al. [8] also reported successful outcomes in 84.9% of their patients with nasolacrimal duct obstruction (NDO). But it is also found that a higher rate of postoperative complications such lacrimal bleeding and/or palpebral edema as compared to previous laser dacryoplasty [9]. Laser dacryoplasty for nasolacrimal recanalization has been in use for more than two decades [10]. The lasers employed include Nd-YAG, THC:YAG, Erbium:YAG, and the Diode laser [10]. The laser probes can be passed through the working channel into the NLD and then can be directly applied to the obstructed tissue with an end-on dacryoendoscopic visualization [9]. Others reported good outcomes in only 66.7% but with lesser rates of lacrimal bleeding and postoperative edema [8]. Electrocautery-based techniques have also been proposed for NLD recanalization in cases of NDO and the probe used is made of copper silver alloy of 140 mm length and 1.2 mm diameter with a 2 mm round tip [9]. Previously, the benefits of using lacrimal canaliculi laser and successful outcomes in 93% of their cases were demonstrated [11]. Agrawal et al. [12] used a 20-gauge bipolar probe connected to a 7-W diathermy console for the NLD recanalization in reported successful outcomes in 92.7%.
Congenital or pediatric NDO
Regarding congenital NDO (CNDO), the diameter of DE should be relatively small and the image quality is limited [13]. So, high-resolution DE only enables a clearer visualization of CNDO, leading to an improved understanding of their features [13].
They perforated the dimple and attempted to remove the white fibrotic tissue using DE (FT-203F MD10, FiberTech) from an 8-year-old boy with Down syndrome and epiphora and mucopurulent discharge, whose lumen was found to be covered with white widespread fibrotic tissue [13].
Recently reported studies showed that a significant percentage of pediatric dacryocystorhinostomy (DCR) cases had an etiology other than typical CNDO [14]. Direct endoscopic probing CNDO cases using DE confirmed the various sites of obstruction and conditions such as edematous thickening of the mucosa of the NLD and fibrous tissue because of chronic inflammation in the children who were 14 to 74 months of age [15]. The sites of obstruction included the punctum, canaliculus, sac, NLD as well as the valve of Hasner [15].
As for recalcitrant CNDO, DE-guided probing was effective with the success rate of 97.1% a year after surgery [16]. Among the cases that failed at other hospitals, they comprised the formation of false passages in the middle area of the NLD, as well as slit-like adhesion at the distal end of the NLD [16]. The recalcitrant CNDO secondary to repeat probing [17–19], late probing [19–22], and complex CNDO [23–26], with reported success rates of 25% to 61%, 33% to 89%, and 33% to 52%, respectively, could be compared with the study using DE. Complex CNDO includes generalized blockage of the duct, sac fibrosis, NLD dysplasia or aplasia, or bony obstruction.
Regarding the false passage, they exhibited a series of cases with three false passages on the temporal side and two on the anterior side [16]. The distal part of the NLD may be curved inward [27] and form a more obtuse angle with the forehead in infants compared with that in adults [28]. These features occasionally complicate blind procedures and lead to false passage formation instead of rupturing the obstructive membrane [16].
Complex CNDO accounted for up to 30% of congenital nasolacrimal duct obstruction cases subjected to probing [24–26]. Regarding the complex CNDO, 18 consecutive DE in 16 patients were studied to analyze the success rate and effectiveness of early DE (PolyDiagnost) with insertion of an auto-stable silicone probe (Monoka, FCI Ophthalmics) in the 1st year of life [29]. They showed over all higher success rate of DE, underlining the effectiveness of DE-guided probing in very young infants, but a poor outcome after probing and syringing, and all patients with severe dacryocystitis.
In failed probing cases of dacryocele preoperative imaging may be helpful but carries a potential risk of radiation toxicity in CNDO [27]. DE showed injection and hemorrhages on the inner wall of the cyst in CNDO, and when cystic distention was observed beneath the inferior turbinate, marsupialization of the cyst could be successfully performed for the cases at age 43 days [30]. DE-assisted nasal endoscopic marsupialization for congenital dacryocystocele were studied with localization of a deflated intranasal cyst after a n initial incision of the cyst [31]. They made a cruciate incision on the medial wall of the dacryocystocele using a sickle knife for marsupialization [32,33]. In membranous CNDOs older than 1 year with history of initial probing failure, direct DE-assisted incision of Hasner’s valve under nasoendoscopy was introduced. The dilated membrane was pruned using mucosa cutters or scissors until there was a fissure at Hasner valve wide open [34].
Transcanalicular endoscopic dacryoplasty showed the success rate of probing by subsequent silicone tube intubation (SI) was 100% using the DE and an 18-gauge intravenous catheter fitted onto the endoscope probe [35]. When divided the CNDO into two categories: the simple and complicated types, the complicated type was divided into two subtypes, the stenosis and fibrosis subtypes [35]. The complex type of CNDO was reported with an incidence of 2.2% to 3.6% in children under 24 months of age and of 20% to 57% in children between 24 and 64 months of age [21], but they suggested that many of the cases resulted from misguided blind probing [35]. To highlight the factors responsible for failure with successful recanalization obviating the need for DCR, 13 refractory CNDOs was studied. All cases had an associated intact membrane over the end of NLD that had been perforated during the previous intervention as per records except four cases of bony NLD dysgenesis. And seven cases could be successfully recanalized using DE [36].
Canalicular stenosis
DE can also help with the diagnosis and localization of the lesions within the canaliculus, as well as evaluation of degrees of canalicular stenosis [1]. The accumulated concretions, together with the mucopurulent discharge, are responsible for a self-perpetuating cycle of canalicular stasis and infection for years [37]. Cobblestone-like intracanalicular granulation tissues [38] or fibrous tissues caused by Campylobacter concisus were also removed repeatedly until the confirmation of their complete clearance under DE [39,40].
Another study enrolled 42 primary canaliculitis patients with canalicular dilatation who underwent canaliculoplasty including canaliculotomy, curettage of canalicular contents with stent placement using DE [41]. They found polypoid changes of the canalicular mucosae in 81% of patients, mainly involving the distal part of the horizontal canaliculus, in which the microbiologic cultures were negative. The long-lasting inflammation was more likely contributed by the concretions and thick mucopurulent discharge compared to the infection of bacteria itself.
DE shows edematous canalicular mucosa with areas of hemorrhages and circumferential narrowing of the lumen with no evidence of discharge or dacryoliths [42]. Canalicular obstructions with associated NDO have also been described in cases of cicatrizing conjunctival diseases like ocular cicatricial pemphigoid and lichen planus [42].
Bicanalicular cystic swellings associated with punctal agenesis were examined by DE [43] and the cystic lesions could be recanalized under DE-guided bicanalicular intubation sparing excision of the cyst wall [44]. DE examinations in five patients with a peripunctal tumor were useful to check for patency of the LDS, which showed its patency [45]. A patient with upper canalicular squamous papilloma treated with DE-guided transcanalicular intralesional and topical interferon alpha was presented [46].
The onset of epiphora ranged from 2 to 8 months after the initiation of chemotherapy [47]. Using DE observation, the incidence of lacrimal obstruction or stenosis associated with S-1 was reported 10% of patients like punctal stenosis, canalicular obstruction or stenosis [47]. Pemetrexed used in the management of mesotheliomas and non–small cell lung carcinomas is also known as drug-inducing punctal and canalicular stenosis [48]. Sharma et al. [48] introduced the uninflated balloon segment of the coronary catheter into the punctum and the canaliculi, progressively dilating the canaliculus until a hard stop was felt using DE.
Canalicular obstructions can often be a source of therapeutic challenges [49]. A drug-eluting coronary stent, which was collapsed around a balloon at the tip of a catheter, was inserted into the canaliculus under DE guidance and would act as a scaffold to direct epithelialization and prevent fibrosis by slowly releasing an antiproliferative drug, such as corticosteroid [49].
Acquired NDO
Regarding primary acquired NDO, DE allows for a comprehensive investigation of the lacrimal path and can help to understand phenomena that are not confirmed by other tests [50]. DE presented that about 73% of the cases were obstructed in the LDS from NDO patients diagnosed to be normal in the dacryocystography (DCG) [50]. There are various common causes of NDO including secretory changes like mucous or stones and granulation as well as the structural changes like membrane, stenosis and fibrosis inside the LDS [51]. Furthermore, DE evaluation could demonstrate the specific pathologic findings such as submucosal varices at the sac to the duct junction in a patient presenting with bloody tears [52]. General obstructive findings including common canalicular polyps with stenosis, fibrosis in the lacrimal sac, and stenosis of the NLD were revealed and recanalized using DE in a tearing patient treated with radioiodine therapy for thyroid carcinoma [53]. NDO was also successfully treated with DE-guided SI after chemotherapy or radioactive iodine therapy [54].
In the treatment of NDO, there are SI and DCR [50]. The surgical success rate of SI using DE was reported higher than that of conventional SI, since the recanalization could be achieved by identifying the internal structure of the LDS [50]. Individuals with narrow interorbital distances and wide noses would show the greatest lateral divergence along the descending course of the NLD, which should be taken into consideration as the outward type for the surgeon to perform the surgery [55].
A total of 149 cases with NDO were managed and reported using DE and nasal endoscopy after local anesthesia [56]. Higher NDO comprising inflammation of sac and duct with large membranous NLD at sac-duct junction was diagnosed from 73.2% of the patients, compared to the lower NDO of membranous obstruction around the Hasner valve [56]. Injection of prednisolone acetate ophthalmic ointment into the lacrimal passage have successfully treated the complication of granulation tissue formation occurred 10% of NDO patients followed by DE for 12 months [57]. However, lipogranuloma formation would be also considered after injection of the ointment into the subepithelium layer, which can occur after false passage by blind probing or after laser canaliculoplasty [58]. Inferior meatal dacryorhinotomy for lower NDO were treated assisted by DE, nasal endoscope, and radiofrequency scalpel with the success rate of 87.5% [59]. Conventional nasal endoscope or nasal examination is still effective for the detection of submucosal insertion, since it enables detection of the direction and site of submucosal insertion at the sac and NLD [59]. Recently, DE-assisted recanalization and balloon dilatation of the NLD using the Ophtacath Lacrimal Duct Balloon Catheter (FCI Ophthalmics) were reviewed with 95.6% anatomic and functional patency test in 115 patients [60].
Regarding long-term efficacy of DE-guided SI in 74 NDOs with the mean age of 60.3 ± 10.0 years, the success group consisted of 89.2% of patients during the median follow-up period of 58 months. Compared to the eyes with preoperative irrigation test of partial passage, the eyes with no passage were associated with a lower success rate [61]. Postoperative inflammation was also associated with a lower success rate and the age could be an important factor of the occurrence of inflammation [61], while dacryolith could be a predictive factor for successful DE-guided SI [62].
Regarding the patients who failed treatment with conventional SI, stenosis was identified as the most common factor and the NLD was the most frequent level of obstruction, followed by the lacrimal sac, canaliculus, and inferior meatus [50]. DE enables real-time observation of the lumen of the LDS, thus facilitating management of pathological lesions including false passages. With this technique, we could be better able to make customized treatment of patients with false passages, with a safer and more effective results leading to the success of DE-guided SI in NDO [63]. Since DE examination in failed DCR could directly and clearly visualize the obstruction site at the rhinostomy based on the relationship between the remnant lacrimal sac mucosa and the obstruction tissue [64], the use of DE is very helpful to repair of failed DCR cases [65].
Imaging inside LDS using DE
Thirty-one patients with clinically suspected NDO who underwent MR-DCG and DE with subsequent surgical procedure were studied [66]. Using heavily T2-weighted fast spin echo sequence in the coronal and axial planes after normal saline drops into the conjunctival sacs, the overall accuracy of MR-DCG in depicting stenosis or obstruction was 84%. MR-DCG could not examine the coexistent stenosis or obstruction at the canalicular level proximal to the lacrimal sac, due to retained fluid collection of thickened mucus in the nasolacrimal duct.
To assess the diagnostic information provided by DE in patients with epiphora, 34 patients with epiphora were prospectively studied and followed by digital subtraction DCG [67]. Among the 30 lacrimal pathways that were normal by DCG, obstruction was revealed in 22 cases by DE, with 11 cases in the common canaliculus. It showed that DE allowed for comprehensive investigations of the lacrimal pathway and can help explain unidentified factors associated with lacrimal pathway obstruction in patients with epiphora.
To facilitate the analysis of lacrimal conditions using high-definition DE (HDD), a study found out that air-in sufflated HDD had better image quality than saline-irrigated HDD [68]. The vascular pulsation and canalicular-sac movement during blinking became visible at lower air pressures (5 kPa), and higher air pressures (15–20 kPa) enabled a better, but avascular, image due to ductal extension. Even though saline irrigation should have an insufficient effect on turbidity removal in the visual field, the higher the air pressure and the higher the risk of emphysema in the case of a perforated lacrimal mucosa. Recently the DE images were reported to process and upgrade using comb-removal, increase in the number of pixels and image-sharpening algorithms [1,69].
Other applications
As for the various LDS conditions, the biopsy specimens could be obtained by scraping the lesion by sheath advancement of DE [70]. From the patients with recurrent NDO, stratified epithelium and a mixed inflammatory cell infiltrates were identified suggesting squamous metaplasia of the usual respiratory epithelium [70]. Similar findings were also reported a subepithelial mixed inflammatory cell and fibroblast infiltration in the early phase, and squamous metaplasia and subepithelial fibrosis in the chronic phase (Fig. 2) [71].
Regarding the mechanical movement of the lacrimal sac, the lateral wall of the sac was observed to move with pressure changes flexibly using DE [72]. Under positive pressure, the wall moved outward, but it moved inward under negative pressure. And the point 3 mm below the common internal ostium corresponded to the lower margin of the Horner muscle [73]. The movement of the lacrimal sac wall with intra sac pressure changes was confirmed to contribute to lacrimal drainage of the sac by DE [72]. The extent of movement was more dramatic in the common canalicular portion than the proximal canalicular portion through the examination of the movement of the lacrimal canalicular wall with DE in relation to positive and negative pressure changes [74].
However, there is also a need for better evidence-based understanding on the safety and optimal utilization of endoscopy for patient care during COVID-19 pandemic [75]. When screening of the patients for COVID-19 symptoms, laboratory tests where needed, adherence to specific operating room guidelines, and personal protective equipment should facilitate performance of DE during the COVID-19 pandemic for the future [75].
Conclusion
DE allows direct visualization of the internal condition of the lacrimal passage, to directly diagnose the site of obstruction with accuracy in NDO [56,76–78]. It enables a stenosis to be identified precisely and offers detailed examination of the anatomy and underlying pathologies of the LDS and of the nose such as ectasia of the lacrimal sac, post–saccal stenosis and dacryoliths showed [5]. So far, the most suitable candidates for DE-guided procedures would be focal or segmental NDO, dacryolith, or concretions in a patent system, stenosis, and obstruction anywhere in LDS [1]. But the limitations of the DE included expensive instrumentation, steep learning curve, ill-understood etiopathogenesis, postoperative healing, frequent restenosis, lower success rates, high costs, and lack of robust long-term outcomes [1].
Nevertheless, the fast rehabilitation, minimally invasive character, low complication rate, and short treatment time make the intervention very attractive as a first-step procedure even for tearing patients [5]. And the associated chromatic aberration, diffraction, and difficulties in deep-set eyes are under development to overcome their potential limitations. However, skepticism could be well justified with regards to NLD recanalization [9]. The surgical techniques need to be upgraded to resolve the limitations in order to replace DCR in the management of NDO, for which there is a lot of hope [9].
Acknowledgements
None.
Notes
Conflicts of Interest: Helen Lew is a member of the Editorial Board of the Korean Journal of Ophthalmology since 2015. However, she was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Funding: None.