Tractional Retinal Detachment in Eyes with Vitreous Hemorrhage and Proliferative Diabetic Retinopathy and Posterior Vitreous Detachment in Fellow Eye
Article information
Abstract
Purpose
To predict the presence of tractional retinal detachment (TRD) in eyes with dense vitreous hemorrhage (VH) and proliferative diabetic retinopathy (PDR) by evaluating the status of posterior vitreous detachment (PVD) in fellow eyes using optical coherence tomography (OCT).
Methods
A total of 44 eyes from 22 patients who underwent vitrectomy due to dense VH with PDR were enrolled. Using OCT, the PVD status in the fellow eye was divided into two groups (incomplete and complete PVD). The incomplete PVD group included eyes without PVD and eyes with partial PVD. B-scan ultrasonography was performed on eyes with dense VH to evaluate the presence of TRD. Both OCT and B-scan images were reviewed by four ophthalmologists (two novices and two experienced), and the interobserver agreement was evaluated.
Results
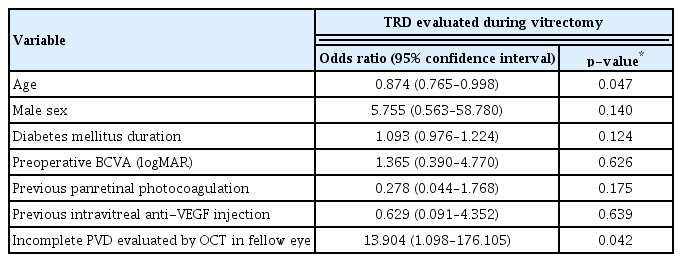
There was a difference in the interobserver agreement regarding the presence of TRD in eyes with dense VH evaluated by B scan between novice and experienced ophthalmologists (novice, κ = 0.421 vs. experienced, κ = 0.814), although there was no difference between novice and experienced ophthalmologists in the interobserver agreement regarding the status of PVD in the fellow eye evaluated by OCT (novice, κ = 1.000 vs. experienced, κ = 1.000). All observed TRD during vitrectomy occurred in eyes with incomplete PVD in the fellow eye. Logistic regression analysis revealed a statistically significant relation between TRD and the age of the patient (odds ratio [OR], 0.874; p = 0.047), and between TRD and incomplete PVD in the fellow eye evaluated by OCT (OR, 13.904; p = 0.042).
Conclusions
Evaluation of the PVD status in the fellow eye using OCT may be a useful predictor for detecting the presence of TRD in eyes with dense VH and PDR.
Diabetic retinopathy (DR), one of the most common microangiopathic complications of diabetes mellitus (DM), is a major cause of vision loss worldwide, including Korea [1]. Hyperglycemia may lead to retinal microinfarctions and capillary nonperfusion, thereby causing ischemic microcirculation disorders in the retina leading to neovascularization (NV) [2,3].
The advancement of NV in DR can lead to vitreous hemorrhage (VH) or fibrovascular proliferation, resulting in tractional retinal detachment (TRD), which significantly contributes to severe vision loss [4,5]. Given the crucial role of TRD in the visual prognosis and management of eyes with proliferative DR (PDR), it is essential to conduct meticulous screening for its presence.
It is known that the vitreous attachment to the retina is a crucial factor for the development of retinal NV [6,7]. Posterior vitreous detachment (PVD) is a separation between the posterior vitreous cortex (PVC) and the internal limiting membrane of the retina, resulting from weakening of the adhesion between the vitreous cortex and the internal limiting membrane [8]. PVD is associated with the progression of PDR [6] and the PVD status in eyes with diabetic vitrectomy has been reported to be a prognostic factor for functional outcomes [7]. In addition, PVD status is considered one of the contributing factors for the development of TRD in eyes with DR [6,7].
Traditionally, B-scan ultrasonography (USG) has been used to assess the status of PVD. However, with the advancement of spectral domain optical coherence tomography (SD-OCT), better visualization of the vitreoretinal interface became possible [9]. Previous studies have demonstrated that SD-OCT can provide high-resolution images of PVC and may serve as a valuable tool in evaluating the status of PVD [10–13]. Moon et al. [14] compared the diagnostic abilities of B scans and OCT when evaluating the status of PVD and found that OCT could accurately evaluate the status of PVD.
Despite the advantages of SD-OCT in visualizing the vitreoretinal interface, its utility in evaluating the posterior segment, including the status of PVD and the presence of TRD, may be limited in eyes with PDR and dense VH due to vitreous opacification. In such cases, B scans are commonly used to assess the posterior segment. However, relying solely on B scans for the evaluation of the posterior segment may lead to missed detection of detailed retinal findings, such as posterior hyaloid detachment [15]. In addition, in eyes with more complex findings, such as TRD, differences between B-scan findings and actual operative findings have been reported [16].
As mentioned earlier, the development of TRD in eyes with PDR is closely associated with the status of PVD. In addition, previous studies have reported that PVD frequently develops in the fellow eye within 6 months to 2 years after its development in the first eye [17–19]. Accordingly, our study aimed to investigate the correlation between the presence of TRD evaluated during vitrectomy in eyes with dense VH and the status of PVD in the fellow eye assessed by OCT. Additionally, we evaluated the interobserver agreement and the influence of ophthalmologist experience (novice vs. experienced) in assessing the presence of TRD in eyes with dense VH using B scans, and in the evaluation of the status of PVD in the fellow eye by OCT.
Materials and Methods
Ethics statements
This study was reviewed and approved by the Institutional Review Board of Kangdong Sacred Heart Hospital (No. 2022-11-002). The study adhered to the tenets of the Declaration of Helsinki, and the requirement for informed consent was waived by the Institutional Review Board of Kangdong Sacred Heart Hospital due to the retrospective nature of the study.
Subjects
In this retrospective observational study, we reviewed the medical records of patients who underwent vitrectomy at the Department of Ophthalmology, Kangdong Sacred Heart Hospital (Seoul, Korea) between March 1, 2015 and August 31, 2019. Patients who underwent vitrectomy in one eye for dense VH (defined as grade 4 by Nussenblatt standardization for vitreous haze grading) associated with PDR were enrolled [20]. All patients had PDR without VH in their fellow eye. The exclusion criteria were as follows: (1) patients with any retinal diseases other than DR, (2) patients with a history of vitrectomy in either eye, (3) those with neovascular glaucoma in either eye, and (4) patients aged <40 years due to the low prevalence of PVD in younger patients [19,21,22]. Ultimately, 22 patients were included in the study.
All patients underwent comprehensive ophthalmic examinations, including measurement of best-corrected visual acuity (BCVA), slit-lamp biomicroscopy, detailed fundus examination, wide-field fundus photography (Optos 200Tx, Optos), SD-OCT (Spectralis HRA & OCT ver. 1.10.2.0, Heidelberg Engineering), and B-scan USG (Aviso, Quantel Medical). Demographic factors, such as age, sex, duration of DM, usage of insulin, glycated hemoglobin (HbA1c), lens status, panretinal photocoagulation (PRP), and intravitreal anti–vascular endothelial growth factor therapy injection in both eyes were obtained from medical records. After the examinations, all patients underwent vitrectomy in eyes with dense VH, and the retina was evaluated after the removal of the dense VH during surgery.
B-scan USG evaluation of eyes with VH
B scans were performed on eyes with dense VH by a single experienced examiner using a 10-MHz focused probe. The eyelids of the patient were kept open, and the probe was placed directly on the ocular surface behind the limbus with 2.5% methylcellulose as a coupling gel while in the supine position under topical anesthesia. The probe was placed at the 3-, 6-, 9-, and 12-o’clock positions, and images from across the optic disc were obtained. The decibel gain was adjusted for each examination to obtain the best images of ocular structures.
B-scan images were independently reviewed by four ophthalmologists (two novice residents [CWJ, YA] and two experienced professors [KLK, YDK]) who were blinded to patient information.
SD-OCT evaluation in the fellow eye
SD-OCT of the fellow eye was performed by an experienced examiner before vitrectomy in eyes with dense VH. We performed a 30°-line transverse scan taken with a 7° tilt, traversing both the optic disc and fovea simultaneously. Images with an SD-OCT quality score <20 were excluded from the analysis.
Evaluation of PVD in the fellow eye
The status of PVD in the fellow eye was evaluated using transverse scan images and analyzed with reference to a previous study [14]. We categorized PVD status into three grades: no PVD, partial PVD, and complete PVD. Absence of PVD and partial PVD were defined as incomplete PVD (Fig. 1A–1E).
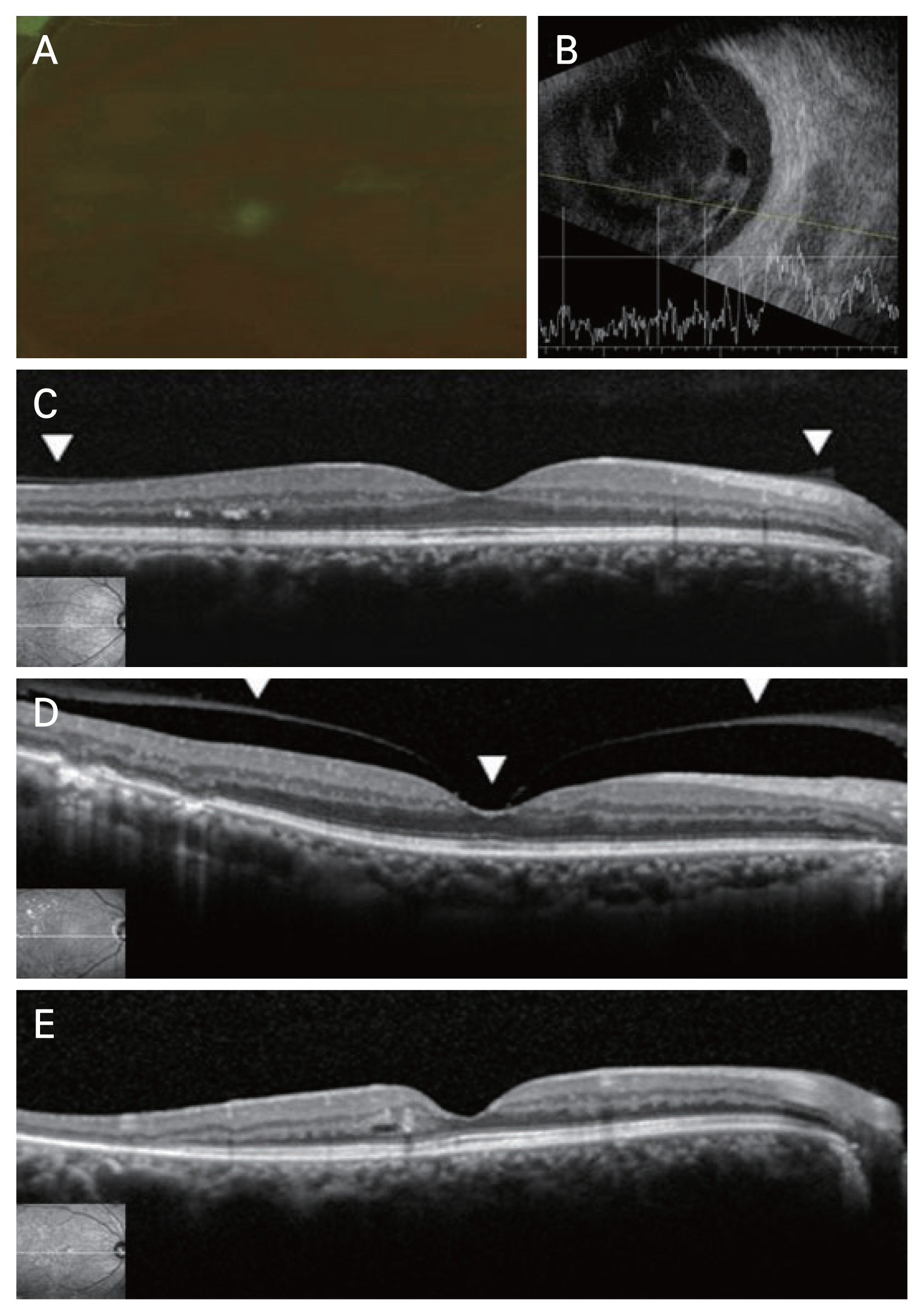
Representative images of wide-field fundus photography and B-scan ultrasonography in eye with dense vitreous hemorrhage (VH) and tractional retinal detachment (TRD) along with representative images of spectral domain optical coherence tomography (SD-OCT) in the fellow eye according to the status of posterior vitreous detachment (PVD; no, partial, and complete). (A) Due to dense VH caused by proliferative diabetic retinopathy, the posterior segment cannot be evaluated in wide-field fundus photography. (B) TRD is observed in the eye with dense VH at B scan. (C) Complete attachment of posterior vitreous cortex (PVC; arrowheads) at perifoveal and parafoveal area evaluated by SD-OCT is defined as no PVD. (D) Partial detachment of PVC (arrowheads) at parafoveal area evaluated by SD-OCT is defined as partial PVD. (E) Absence of observable attached PVC at macular area evaluated by SD-OCT is defined as complete PVD.
In the transverse scan image, no PVD was defined as complete attachment of the PVC to the perifoveal and parafoveal areas, fovea, or optic disc. Patients with a PVD limited to outside the parafoveal area (2,500 μm in diameter) were also considered to have no PVD, as we expected that this subtle PVD would not be detected by B scan. Partial PVD was defined as detached PVC observed within the parafoveal area (2,500 μm in diameter). Complete PVD was defined as the absence of observable attached PVC in the macular or optic disc areas. OCT images were reviewed by the same four ophthalmologists blinded to patient information.
Statistical analysis
Interobserver agreement for the presence of TRD in eyes with dense VH evaluated by B scan and the status of PVD in fellow eyes evaluated by SD-OCT was calculated using the κ correlation. K is a measure of the difference between the observed and expected agreement. The following criteria were used for the interpretation of κ-values: almost perfect (0.81–1.00), substantial (0.61–0.80), moderate (0.41–0.60), fair (0.21–0.40), slight (0.01–0.20), and poor (<0) agreement [23].
We divided patients into two groups (incomplete PVD [including no and partial PVD] and complete PVD) according to the status of PVD in the fellow eye evaluated by SD-OCT. Clinical characteristics and intraoperative findings between the two groups were compared using the Mann-Whitney test and Fisher exact test. Logistic regression analysis was performed to evaluate the statistical significance of relation between the presence of TRD evaluated during vitrectomy in eyes with dense VH and variables including the clinical characteristics and status of PVD in the fellow eye evaluated using SD-OCT. We examined the variance inflation factors (measurement for the degree of collinearity) of the variables for the regression analysis. All statistical analyses were performed using the IBM SPSS ver. 27.0 (IBM Corp). Statistical significance was defined as p < 0.05.
Results
In our study, 44 eyes (22 VH eyes and 22 fellow eyes) from 22 patients who underwent vitrectomy for PDR were included. The mean age of the patients was 57.8 ± 9.4 years, and 18 patients (81.8%) were male. The mean duration of DM was 16.8 ± 10.2 years and nine patients (40.9%) used insulin for control of DM. The mean HbA1c was 7.5% ± 1.3%. The mean preoperative BCVA was 2.3 ± 0.7 logarithm of the minimum angle of resolution (logMAR) in eyes with dense VH eyes and 0.5 ± 0.5 logMAR in the fellow eyes. In eyes with dense VH, five eyes (22.7%) were pseudophakic, eight eyes (36.4%) had PRP, and 10 eyes (45.5%) had a history of intravitreal anti–vascular endothelial growth factor (anti-VEGF) injection (Table 1).
Interobserver agreement for the status of PVD in fellow eyes evaluated by SD-OCT was perfect between the two novice ophthalmologists (κ = 1.000; 95% confidence interval [CI], 1.000–1.000) and between the two experienced ophthalmologists (κ = 1.000; 95% CI, 1.000–1.000) (Table 2). However, the interobserver agreement for the presence of TRD in eyes with dense VH evaluated by B-scan USG was moderate between the two novice ophthalmologists (κ = 0.421; 95% CI, 0.100–0.742) and almost perfect between the two experienced ophthalmologists (κ = 0.814; 95% CI, 0.573–1.055) (Table 3). TRD evaluated during vitrectomy was observed in seven eyes (31.8%). All seven patients with TRD had incomplete PVD, as evaluated by OCT in their fellow eyes (Table 4).

Interobserver agreement between two novice ophthalmologists and two experienced ophthalmologists for the status of PVD evaluated by spectral domain optical coherence tomography in fellow eye

Interobserver agreement between two novice ophthalmologists and two experienced ophthalmologists for the presence of TRD evaluated by B-scan ultrasonography in eyes with dense vitreous hemorrhage

Presence of TRD evaluated during vitrectomy in eyes with dense vitreous hemorrhage and status of PVD evaluated by spectral domain optical coherence tomography in fellow eye
There were no significant differences in age, sex, duration of DM, usage of insulin, HbA1c, preoperative BCVA, lens status, previous history of PRP, or intravitreal anti-VEGF injection according to the status of PVD in the fellow eye ( p = 0.649, p > 0.999, p > 0.999, p = 0.609, p = 0.842, p = 0.842, p = 0.548, p = 0.613, and p > 0.999, respectively). The presence of TRD evaluated during vitrectomy and gas or silicone oil tamponade was observed more in eyes with incomplete PVD in the fellow eye, although the difference was not significant (p = 0.245 and p = 0.115, respectively) (Table 5).
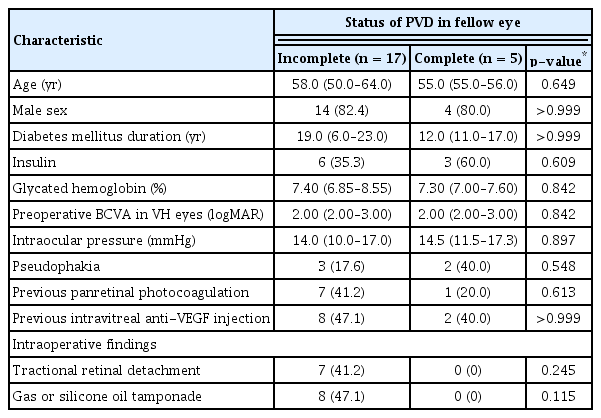
Comparison of clinical characteristics and intraoperative findings in eyes with VH according to the status of PVD in the fellow eye evaluated by optical coherence tomography
The variance inflation factors of all variables in our analysis were less than 1.5. The logistic regression analysis revealed that sex, duration of DM, preoperative BCVA, previous history of PRP, and intravitreal anti-VEGF injection were not statistically significant in the presence of TRD evaluated during vitrectomy (p = 0.140, p = 0.124, p = 0.626, p = 0.175, and p = 0.639, respectively). However, there was a significant relationship between age and the presence of TRD during vitrectomy (odds ratio [OR], 0.874; 95% CI, 0.765–0.998; p = 0.047). Furthermore, there was a significant relationship between incomplete PVD in the fellow eye evaluated by SD-OCT and the presence of TRD evaluated during vitrectomy (OR, 13.904; 95% CI, 1.098–176.105; p = 0.042) (Table 6).
Among the 17 eyes with incomplete PVD in fellow eyes evaluated by SD-OCT, the status of PVD evaluated during vitrectomy was not documented in three eyes. Meanwhile, PVD induction was done during vitrectomy in all remaining 14 eyes that had incomplete PVD in fellow eyes evaluated by SD-OCT. Among the five eyes with complete PVD in fellow eyes evaluated by SD-OCT, PVD induction during vitrectomy was done in only one eye.
Discussion
In our study, we observed a discrepancy in the interobserver agreement for the detection of TRD evaluated using B scans in eyes with dense VH between novice and experienced ophthalmologists. However, there was no disparity in the interobserver agreement for the assessment of PVD status using OCT in fellow eyes between novice and experienced ophthalmologists. Furthermore, TRD evaluated during vitrectomy was observed in eyes of patients with incomplete PVD in the fellow eye. The logistic regression analysis demonstrated a significant correlation between TRD and the age of the patient, as well as TRD and incomplete PVD status evaluated using OCT in the fellow eye.
We analyzed SD-OCT images of the fellow eye of patients with PDR who had dense VH in one eye that obscured the posterior pole and thus hindered the prediction of the exact condition of the eye with dense VH. Our approach is based on two concepts. First, PVD typically manifests in one eye during middle or older age, while the other eye usually develops PVD within 6 months to 2 years [17–19]. Second, the concurrence of strong vitreoretinal adhesion and shrinkage of the vitreous occurs at the PVC in eyes with incomplete PVD compared to those with complete PVD [6].
PVD status is an important prognostic factor in eyes with PDR because vitreoretinal and vitreopapillary traction can cause serious damage to the retina and optic nerve head [24]. Vitreoretinal adhesion in DR is associated with membrane proliferation, NV, and TRD, resulting in poor visual outcomes [7]. In addition, incomplete PVD with thick PVC was reported to be an important factor in DR progression [6].
OCT is a useful technique for evaluating PVD status. Moon et al. [14] reported that OCT had a comparable diagnostic value in staging the status of PVD compared to B scans. OCT showed higher interexaminer agreement and was more independent according to the investigator than B scans. In particular, by adding peripapillary scan images to transverse scan images, the status of PVD can be diagnosed more accurately using OCT.
Although B scan is a widely used conventional technique to evaluate vitreoretinal status, including TRD in patients with dense VH, it lacks sufficient resolution to definitively identify the presence and degree of TRD [16,25]. Compared with OCT, the imaging protocol and examination method of B scans are less specific and more dependent on the skill of the examiner. Thus, it is necessary for the examiner to recognize any suspected retinal pathology, such as TRD or PVD, and obtain detailed imaging of the suspected site. In our study, the interobserver agreement for the presence of TRD evaluated by B scan in eyes with dense VH was almost perfect between two experienced ophthalmologists, but moderate between two novice ophthalmologists. Conversely, the interobserver agreement for the status of PVD evaluated by OCT in the fellow eye was perfect across all four ophthalmologists (two novices and two experienced). These findings suggest that interpretation of B-scan results may vary among novice ophthalmologists with less experience, while evaluation of PVD status using SD-OCT is relatively consistent regardless of the level of experience.
In our study, there was a significant relationship between age and the presence of TRD detected during vitrectomy in eyes with dense VH. This implies that the risk of PDR progression and TRD formation, a vision-threatening complication in patients with DR, is lower in older patients. This result is consistent with the findings of previous studies [26,27]. Porta et al. [26] reported that younger age and early diagnosis of DM were risk factors for PDR progression, while Davis et al. [27] found that younger age was a risk factor for the progression to high-risk PDR. Although we excluded patients under 40 years of age due to the low prevalence of PVD in this age group, there was a significant relationship between the presence of TRD detected during vitrectomy in eyes with dense VH and younger age. This finding may relate to the higher prevalence of PVD with increasing age [28,29].
Proliferative changes in various vitreoretinal diseases, including PDR, are caused by NV from the retina and optic disc into the vitreous [30]. Thus, the status of PVD is correlated with the progression of proliferative changes in eyes with PDR, and it is possible that PVD status may affect the development of TRD in these eyes. In eyes with partial PVD, strong vitreoretinal adhesions and vitreous shrinkage occur in the PVC. The sites of strong adhesion and leakage of blood components induce shrinkage of the PVC, leading to TRD. In contrast, in eyes with complete PVD, there is no vitreoretinal adhesion, which induces traction between the PVC and the internal limiting layer. Similarly, in our study, assuming that PVD generally occurs at a similar time in both eyes [17–19], the presence of TRD during vitrectomy was observed in patients with incomplete PVD evaluated by OCT in the fellow eye, although with borderline significance. In the logistic regression analysis, there was a significant relationship between the presence of TRD detected during vitrectomy and incomplete PVD in the fellow eye (OR, 13.904; 95% CI, 1.098–176.105; p = 0.042). Thus, based on the two concepts mentioned above and the findings of our study, incomplete PVD evaluated by OCT in the fellow eye may be correlated with the presence of TRD in eyes with dense VH and PDR.
This study has several limitations. First, the sample size was relatively small as we evaluated a limited number of patients who underwent vitrectomy in one eye due to dense VH with PDR and had no signs of VH in the fellow eye. Nevertheless, despite the small sample size, we observed a significant relationship between the presence of TRD detected during vitrectomy and incomplete PVD of the fellow eye evaluated by OCT. Second, retrospective evaluation of B-scan images precluded kinetic evaluation of B scans. Third, we assumed that the PVD status would be similar in both eyes, but it is possible that it might differ between both eyes. Nonetheless, PVD status is generally similar in both eyes as the vitreoretinal interface changes in an age-dependent manner [17–19].
In conclusion, the interpretation of the presence of TRD in eyes with dense VH and PDR using B scans can be influenced by the skill or experience of the ophthalmologist. In addition, incomplete PVD in the fellow eye, as determined by OCT, may be associated with the presence of TRD in eyes with dense VH and PDR. The assessment of PVD status in the fellow eye by OCT may be helpful in predicting the presence of TRD in eyes with dense VH and PDR. Further large studies are needed to clarify the correlation between the TRD in eyes with dense VH and PVD status in fellow eyes evaluated by OCT.
Acknowledgements
None.
Notes
Conflicts of Interest: None.
Funding: This study was supported by the Korean Association of Retinal Degeneration (No. KARD-2016001).