|
 |
| Korean J Ophthalmol > Volume 37(1); 2023 > Article |
|
Abstract
Purpose
To investigate the effect of blunt ocular trauma (BOT) on foveal circulation, and in particular the foveal avascular zone (FAZ), using optical coherence tomography angiography (OCTA).
Methods
This retrospective study consisted of 96 eyes (48 traumatized eyes and 48 nontraumatized eyes) from 48 subjects with BOT. We analyzed the FAZ area of deep capillary plexus (DCP) and superficial capillary plexus (SCP) immediately after BOT and at 2 weeks after BOT. We also evaluated the FAZ area of DCP and SCP in patients with and without blowout fracture (BOF).
Results
There were no significant differences in FAZ area between traumatized and nontraumatized eyes at DCP and SCP in the initial test. In traumatized eyes, the FAZ area at SCP was significantly reduced on follow-up when compared to initial test (p = 0.01). In case of eyes with BOF, there was no significant differences in FAZ area between traumatized and nontraumatized eyes at DCP and SCP on initial test. No significant difference of FAZ area was found on follow-up relative to the initial test, whether in the DCP or SCP. In case of eyes without BOF, there was no significant differences of FAZ area between traumatized and nontraumatized eyes at DCP and SCP in initial test. Also, no significant difference of FAZ area at DCP was found on follow-up test compared to initial test. However, the FAZ area at SCP was significantly reduced in follow-up test compared with that in the initial test (p = 0.04).
Conclusions
Temporary microvascular ischemia occurs in the SCP of patients after BOT. Patients should be warned of transient ischemic changes that may occur after trauma. OCTA can provide useful information regarding the subacute changes in the FAZ at SCP after BOT, even without evident findings of structural damage on fundus examination.
Ocular trauma is a major cause of monocular blindness and visual impairment worldwide [1] and the World Health Organization has reported that globally 55 million people experience serious ocular trauma every year [2]. Blunt trauma was the most common type of ocular trauma [3] and frequently occurs in various situations such as industrial settings, traffic accidents, and assault. Complications that may occur after a blunt ocular trauma (BOT) include traumatic hyphema, commotio retinae, retinal tear, retinal detachment, retinal and vitreous hemorrhage, blowout fractures (BOF), and traumatic optic neuropathy (TON) [4]. If these complications occur, even minor trauma to eye can cause severe visual dysfunction and impairment. Proper evaluation and treatment are needed immediately after the trauma.
Posttraumatic retinal disorders have been described and were attributed to the disruption of choroidal circulation might cause outer retinal damage after BOT [5]. Retinal vascular occlusions secondary to BOT also have been reported [6-10]. According to Long et al. [11], oxygen consumption is increased in eyes after closed-globe BOT, suggesting that BOT may affect retinal circulation. Thus, retinal circulation after BOT is important, but there have only been few studies about the retinal vascular changes after BOT.
Optical coherence tomography angiography (OCTA) is a safe, rapid, noninvasive deep resolved imaging technique that provides en face visualization of the retinal vascular networks by detecting the blood flow in different retinal layers [12-14]. This tool allows for detailed analysis of retinal perfusion damage in macular and peripapillary regions even without evidence of retinal structural loss, such as in several optic neuropathies [15]. OCTA can detect early microcirculatory disturbance [16] and separately evaluate the superficial capillary plexus (SCP) and deep capillary plexus (DCP) in various regions. It also provides a detailed analysis of the foveal avascular zone (FAZ) [13,17], a special part of the retina that maximizes the optical quality of vision [18,19].
According to Montorio et al. [20], BOT can cause temporary reduction in retinal vessel density, which leads to impaired retinal blood perfusion. Retinal perfusion can be estimated indirectly from the FAZ area, and previous studies have reported that the FAZ area is altered by focal ischemia of the retina and retinal microcirculation impairment in patients with branch retinal vein occlusion [21] and diabetic retinopathy [22]. We hypothesized that the patients with BOT may have disturbances in retinal perfusion even without any structural damage. Thus, using OCTA, we can noninvasively evaluate changes in retinal blood perfusion after BOT by comparing the FAZ area between traumatized and nontraumatized eyes. We can also monitor changes over time. Here we focused on changes in posttraumatic retinal perfusion in the absence of structural damage immediately after trauma.
The study was performed adherence with the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of Myoungji Hospital (No. 2021-07-051). The requirement for informed consent was waived due to the retrospective nature of the study.
This retrospective study evaluating imaging technology was conducted at Myongji Hospital from May 2019 to May 2021. The patients who visited the clinic immediately after or within 1 week after trauma and visited the clinic for follow-up 2 weeks after the initial visit were included. Patients who had high myopia (>6 diopters), amblyopia, prior history of systemic disease as diabetes, previous ocular trauma, previous ocular surgery, congenital eye disease, macular or vitreoretinal disease, glaucoma, presence of significant lens opacification, late visit over a week after trauma, or poor quality of OCTA images were excluded from the study. We also excluded patients with penetrating ocular trauma, choroidal rupture, traumatic macular holes, retinal tear or retinal detachment and binocular injured patients by considering changes in FAZ size between normal individuals.
In all patients, a detailed history was obtained to determine the injury type, and the duration between injury and presentation. All patients underwent complete ophthalmic examination, including best-corrected visual acuity (BCVA), intraocular pressure, relative afferent pupillary defect, ocular movement, slit-lamp biomicroscopy, fundus examination, OCT, and OCTA imaging of the macula on both initial and follow-up visits.
All patients, retinal microvasculature was analyzed using a Nidek OCT instrument (RS-3000 Advance 2, Nidek) on both initial and follow-up visits. The RS-3000 spectral domain OCT uses a wavelength of 880 nm with a scan speed of 53,000 A-scans/sec. Scans with poor quality, defined by the following criteria, were excluded: signal quality index <2/5, signal strength index <7/10, poor clarity, residual motion artifacts visible as irregular vessel pattern on the en face angiogram, or off-center macula.
For each eye, a 3 × 3-mm scan centered on the fovea was acquired. OCTA images of the superficial and deep capillary networks were generated separately using the automated software algorithm. Based on the eight regional settings, the SCP layer was defined from the inner limiting membrane to 13 μm above the inner plexiform layer. The DCP layer was defined from 8 μm above the inner plexiform layer to 13 μm above the outer plexiform layer.
We analyzed FAZ area manually with ImageJ (US National Institutes of Health) after setting the scale to 800 × 800 pixels in 3 × 3-mm scan area same as previous study [23,24]. The FAZ area was separately calculated for the SCP area and DCP area and the maximum horizontal and vertical diameters of the FAZ were marked manually as shown in Fig. 1A-1F. All clinical information were masked, and the image analyses were performed independently by two ophthalmologists (LLW and JYL), and the average of the two values was recorded.
Data were analyzed using IBM SPSS ver. 26 (IBM Corp). The FAZ area of traumatized eyes and nontraumatized eyes were compared using Wilcoxon signed rank test and the FAZ area immediately after trauma and 2 weeks after trauma were compared using paired t-test. The correlation of the difference in BCVA and the difference in SCP and DCP FAZ area between immediately after trauma and 2 weeks after trauma was analyzed using Pearson’s correlation test. Reliability analysis was performed by calculating intraclass correlation coefficient (ICC) and interobserver correlation coefficient between graders. A p-value of <0.05 was considered statistically significant. All results were presented as mean ± standard deviation.
Of the 64 patients (128 eyes) who met the inclusion criteria, the following were excluded: four (eight eyes) with binocular injury, eight (16 eyes) who visited the hospital a week after trauma, three (six eyes) had poor OCTA image quality, one subject (two eyes) had a history of glaucoma and retinal surgery. Therefore, 96 eyes from 48 subjects (38 male patients, 79.2%; 10 female patients, 20.8%) were included in the analysis (Fig. 2). The mean age of 48 patients was 40.69 ± 17.37 years. The 17 subjects were right eye injured and 31 subjects were left eye injured. A total of 48 traumatized eyes and 48 nontraumatized eyes were analyzed. Only 27 subjects (54 eyes) visited the hospital for follow-up. A total of 26 eyes (54.2%) were diagnosed as BOF. The mean BCVA of traumatized eyes was 0.06 ± 0.14 logarithm of the minimum angle of resolution (logMAR) at the initial visit and 0.02 ± 0.05 logMAR upon follow-up. The interval between the trauma and the initial visit was 2.46 ± 2.02 days (range, 0-7 days) and the interval between the initial visit and follow-up visit was 14.96 ± 4.66 days (range, 6-28 days). There were no patients with significant retinal and optic nerve complications such as TON or commotio retinae. Detailed demographics were presented in Table 1.
The mean FAZ area of DCP in traumatized eye was 0.54 ± 0.21 mm2 and nontraumatized eye was 0.54 ± 0.20 mm2. The mean FAZ area of SCP in traumatized eye was 0.34 ± 0.13 mm2 and nontraumatized eye was 0.34 ± 0.12 mm2. There were no significant differences of FAZ area between traumatized and nontraumatized eyes at DCP ( p = 0.96) and SCP (p = 0.42) (Table 2).
In traumatized eyes, the FAZ area at SCP was 0.34 ± 0.13 mm2 on initial visit and 0.30 ± 0.12 mm2 on follow-up visit. The FAZ area was significantly reduced on follow-up relative to the initial test (p = 0.013). There was no significant difference in size of the FAZ area in the DCP from initial visit (0.54 ± 0.21 mm2) to follow-up visit (0.53 ± 0.18 mm2, p = 0.197) (Table 2). The amount of FAZ areas between nontraumatized eyes, traumatized eyes, and 2 weeks after in traumatized eyes are presented in Fig. 3A and 3B. The correlation of the difference in BCVA and the difference in SCP and DCP area between immediately after trauma and 2 weeks after trauma was not statistically significant (p = 0.568 and p = 0.906, respectively). The excellent intrarater and interrater correlation was found for all measurement (ICC, 0.98 by LLW; ICC, 0.99 by JYL; ICC, 0.93 between LLW and JYL) [25].
In the subset analysis, we further divided the subjects into eyes with and without BOF and again compared the FAZ area. In the 26 patients with BOF, there was no significant difference in size of FAZ area between traumatized and nontraumatized eyes whether in the DCP (p = 0.758) or in the SCP (p = 0.647). No significant difference in size of FAZ area was found on follow-up versus initial test at DCP (p = 0.326) and SCP (p = 0.162). In the 22 patients without BOF, there was no significant differences in FAZ area between traumatized and nontraumatized eyes at DCP (p = 0.661) and SCP (p = 0.444). Also, no significant difference of FAZ area was found between follow-up and initial in the DCP (p = 0.373). However, the FAZ area in the SCP was significantly reduced on follow-up compared to the initial (p = 0.043) (Table 2).
In the present study, OCTA demonstrated significantly decreased SCP FAZ area in eyes with BOT 2 weeks after trauma. To date, many studies about ocular trauma have been conducted only on BOF or TON. There are few studies about the patients with simple BOT. However, BOT patients who do not have any evident findings on ophthalmic examination sometimes complain of discomfort, including self-reported blurred vision. We hypothesized that a transient subtle perfusion disorder may cause these symptoms. Many studies about posttraumatic retinal perfusion focused on patients with TON [26,27] and commotio retinae [20,28,29]. There are few studies about retinal perfusion in patients with simple BOT. Yalinbas Yeter et al. [30] studied retinal vascular changes and the FAZ area in BOT patients using OCTA. However, they only analyzed the retinal perfusion in patients immediately after trauma, so changes retinal perfusion over time was unknown. Therefore, we aimed to evaluate retinal perfusion by analyzing the FAZ changes in DCP and SCP over a 2-week period after BOT. Yalinbas Yeter et al. [30] found no difference in the FAZ area between the traumatized and nontraumatized eyes 24 hours post-BOT. This was consistent with our own findings. Only at 2 weeks posttrauma was there a significant reduction in FAZ area in the SCP. This indicated that FAZ area in the SCP may be an early and sensitive marker of subacute retinal changes due to trauma.
The mechanism for our findings can be explained by increased oxygen transport and vascular endothelial growth factor (VEGF) expression in the retina after BOT. The neurosensory retina might be stretched by the direct mechanical forces after BOT and this concussive force could be transmitted to the retinal vascular plexus, inducing a vasogenic response, which possibly causes arterial spasm and reduced blood flow [31-33]. These vascular alterations following the trauma, can leads to transient ischemic damage of the retina and leading to increased oxygen transport for retinal repair and clearance of impaired retinal tissue [11]. Previous studies have reported that regulation of retinal oxygenation affects the retinal capillary extent and function [34]. In particular, Petrou et al. [35] reported that the FAZ area reduced in vitrectomy patients due to increased oxygen transport in the ischemic retina. Furthermore, ischemic retina increases VEGF expression, which promotes retinal endothelial cell proliferation [36,37]. Consequently, it seems that increased oxygen transport and VEGF expression in the retina after BOT affects the foveal capillary distribution and leads to a decrease in the SCP FAZ area even there was no structural damage on fundus examination. As a result of our study, the DCP FAZ area after 2 weeks of trauma did not show a statistically significant decrease, but the mean DCP FAZ area decreased. It is thought that additional studies with large patients are needed in the future.
Only in eyes without BOF was there a significant change in size of the SCP FAZ area between initial and follow-up. Some reports have suggested that eyes in blowout injuries are afforded some protection in comparison to eyes in non-blowout injuries [38-40]. Kreidl et al. [41] reported that significant intraocular sequelae, such as retinal detachment, choroidal hemorrhage, and lens dislocation, were higher in mild and moderate ocular trauma without BOF than in severe ocular trauma with BOF and they reported the patients with BOF showed better visual prognosis 1 month after injury compared to patients without BOF. Due to the protective mechanism of orbital fractures, in case of eyes with BOF, it is thought that the FAZ area of SCP did not change after 2 weeks of test compared to the initial test. In case of eyes without BOF, relatively greater damage was absorbed compared to eyes with BOF. As a result, only patients without BOF reduced the FAZ of SCP.
The main limitation of our study was its retrospective nature and cross-sectional design. Also, the lack of further follow-up may obscure the visualization of the changes in FAZ areas during recovery. However, the strength of our study was the relatively sufficient sample size, strictive inclusion criteria and well-planned examination for all patients. Prospective studies with longer follow-up periods are necessary to investigate the role of vascular alterations and FAZ changes in patients with simple BOT who do not have any evident findings on ophthalmic examination.
In conclusion, we demonstrated temporary microvascular ischemia at SCP in patients after BOT. Patients must be warned that transient ischemic changes may occur after trauma. OCTA detects subacute changes in the FAZ at SCP after BOT, even without evident structural damage on fundus examination.
References
3. Liggett PE, Pince KJ, Barlow W, et al. Ocular trauma in an urban population: review of 1132 cases. Ophthalmology 1990;97:581-4.


4. Kim SB, Cho KJ, Cho WH, et al. A statistical observation of non-penetrating ocular injuries. J Korean Ophthalmol Soc 2013;54:938-44.

5. Ishikawa Y, Hashimoto Y, Saito W, et al. Blood flow velocity and thickness of the choroid in a patient with chorioretinopathy associated with ocular blunt trauma. BMC Ophthalmol 2017;17:86.




6. Dalma-Weiszhausz J, Meza-de Regil A, Martinez-Jardon S, Oliver-Fernandez K. Retinal vascular occlusion following ocular contusion. Graefes Arch Clin Exp Ophthalmol 2005;243:406-9.



7. Chong CC, Chang AA. Traumatic optic nerve avulsion and central retinal artery occlusion following rugby injury. Clin Exp Ophthalmol 2006;34:88-9.


8. Umeed S, Shafquat S. Commotio-retinae and central retinal artery occlusion after blunt ocular trauma. Eye (Lond) 2004;18:333-4.



9. Cumurcu T, Doganay S, Demirel S, Cankaya C. Traumatic optic neuropathy and central retinal artery occlusion following blunt ocular trauma. J Clin Med Res 2011;3:55-7.



10. Noble MJ, Alvarez EV. Combined occlusion of the central retinal artery and central retinal vein following blunt ocular trauma: a case report. Br J Ophthalmol 1987;71:834-6.



11. Long C, Wen X, Zhong LX, et al. Oxygen saturation in closed-globe blunt ocular trauma. Biomed Res Int 2016;2016:8232468.




12. Alnawaiseh M, Lahme L, Treder M, et al. Short-term effects of exercise on optic nerve and macular perfusion measured by optical coherence tomography angiography. Retina 2017;37:1642-6.


13. de Oliveira BM, Nakayama LF, de Godoy BR, et al. Reliability of foveal avascular zone measurements in eyes with retinal vein occlusion using optical coherence tomography angiography. Int J Retina Vitreous 2020;6:35.


14. Levine ES, Custo Greig E, Mendonca LS, et al. The long-term effects of anti-vascular endothelial growth factor therapy on the optical coherence tomography angiographic appearance of neovascularization in age-related macular degeneration. Int J Retina Vitreous 2020;6:39.




15. Wang L, Murphy O, Caldito NG, et al. Emerging applications of optical coherence tomography angiography (OCTA) in neurological research. Eye Vis (Lond) 2018;5:11.




16. Takase N, Nozaki M, Kato A, et al. Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina 2015;35:2377-83.


17. Lavia C, Bonnin S, Maule M, et al. Vessel density of superficial, intermediate, and deep capillary plexuses using optical coherence tomography angiography. Retina 2019;39:247-58.



18. Jonas JB, Schneider U, Naumann GO. Count and density of human retinal photoreceptors. Graefes Arch Clin Exp Ophthalmol 1992;230:505-10.



19. Yu DY, Cringle SJ, Su EN. Intraretinal oxygen distribution in the monkey retina and the response to systemic hyperoxia. Invest Ophthalmol Vis Sci 2005;46:4728-33.


20. Montorio D, D’Andrea L, Cennamo G. Retinal vascular features in ocular blunt trauma by optical coherence tomography angiography. J Clin Med 2020;9:3329.



21. Rispoli M, Savastano MC, Lumbroso B. Capillary network anomalies in branch retinal vein occlusion on optical coherence tomography angiography. Retina 2015;35:2332-8.



22. Freiberg FJ, Pfau M, Wons J, et al. Optical coherence tomography angiography of the foveal avascular zone in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 2016;254:1051-8.




23. Magrath GN, Say EA, Sioufi K, et al. Variability in foveal avascular zone and capillary density using optical coherence tomography angiography machines in healthy eyes. Retina 2017;37:2102-11.


24. Samara WA, Say EA, Khoo CT, et al. Correlation of foveal avascular zone size with foveal morphology in normal eyes using optical coherence tomography angiography. Retina 2015;35:2188-95.


25. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979;86:420-8.


26. Gao Y, Li J, Ma H, et al. The retinal vasculature pathophysiological changes in vision recovery after treatment for indirect traumatic optic neuropathy patients. Graefes Arch Clin Exp Ophthalmol 2021;259:3093-105.




27. Mariak Z, Obuchowska I, Ustymowicz A, et al. The role of vascular factors in the development of traumatic optic neuropathy in course of closed head injury. Klin Oczna 2002;104:384-90.

28. Mansour AM, Shields CL. Microvascular capillary plexus findings of commotio retinae on optical coherence tomography angiography. Case Rep Ophthalmol 2018;9:473-8.




29. Papageorgiou E, Voutsas N, Kotoula M, et al. Optical coherence tomography angiography reveals vascular alterations in pediatric commotio retinae. Eur J Ophthalmol 2021;31:NP44-7.



30. Yalinbas Yeter D, Kucukevcilioglu M, Yesiltas YS, et al. Effect of blunt ocular trauma on retinal microvasculature: an optical coherence tomography angiography study. Photodiagnosis Photodyn Ther 2021;33:102147.


31. Mendes S, Campos A, Campos J, et al. Cutting edge of traumatic maculopathy with spectral-domain optical coherence tomography: a review. Med Hypothesis Discov Innov Ophthalmol 2015;4:56-63.


32. Kohno T, Miki T, Hayashi K. Choroidopathy after blunt trauma to the eye: a fluorescein and indocyanine green angiographic study. Am J Ophthalmol 1998;126:248-60.


33. Hashimoto R, Hirota A, Maeno T. Choroidal blood flow impairment demonstrated using laser speckle flowgraphy in a case of commotio retinae. Am J Ophthalmol Case Rep 2016;4:30-4.



35. Petrou P, Angelidis CD, Andreanos K, et al. Reduction of foveal avascular zone after vitrectomy demonstrated by optical coherence tomography angiography. Cureus 2021;13:e13757.



36. Aiello LP, Northrup JM, Keyt BA, et al. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol 1995;113:1538-44.


37. Pe’er J, Shweiki D, Itin A, et al. Hypoxia-induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Lab Invest 1995;72:638-45.

38. Jayamanne DG, Gillie RF. Do patients with facial trauma to the orbito-zygomatic region also sustain significant ocular injuries? J R Coll Surg Edinb 1996;41:200-3.

39. Jayamanne DG, Igillie RF. Orbital blow-out fractures: long-term visual outcome of associated ocular injuries. J Accid Emerg Med 1995;12:273-5.



Fig. 1
Analysis of optical coherence tomography angiography (OCTA) images of superficial capillary plexus (SCP) and deep capillary plexus (DCP) using ImageJ (US National Institutes of Health). Manual outlining of the border of foveal avascular zone. SCP OCTA images of (A) nontraumatized eye, (B) traumatized eye, and (C) traumatized eye 2 weeks after trauma. DCP OCTA images of (D) nontraumatized eye, (E) traumatized eye, and (F) traumatized eye 2 weeks after trauma.
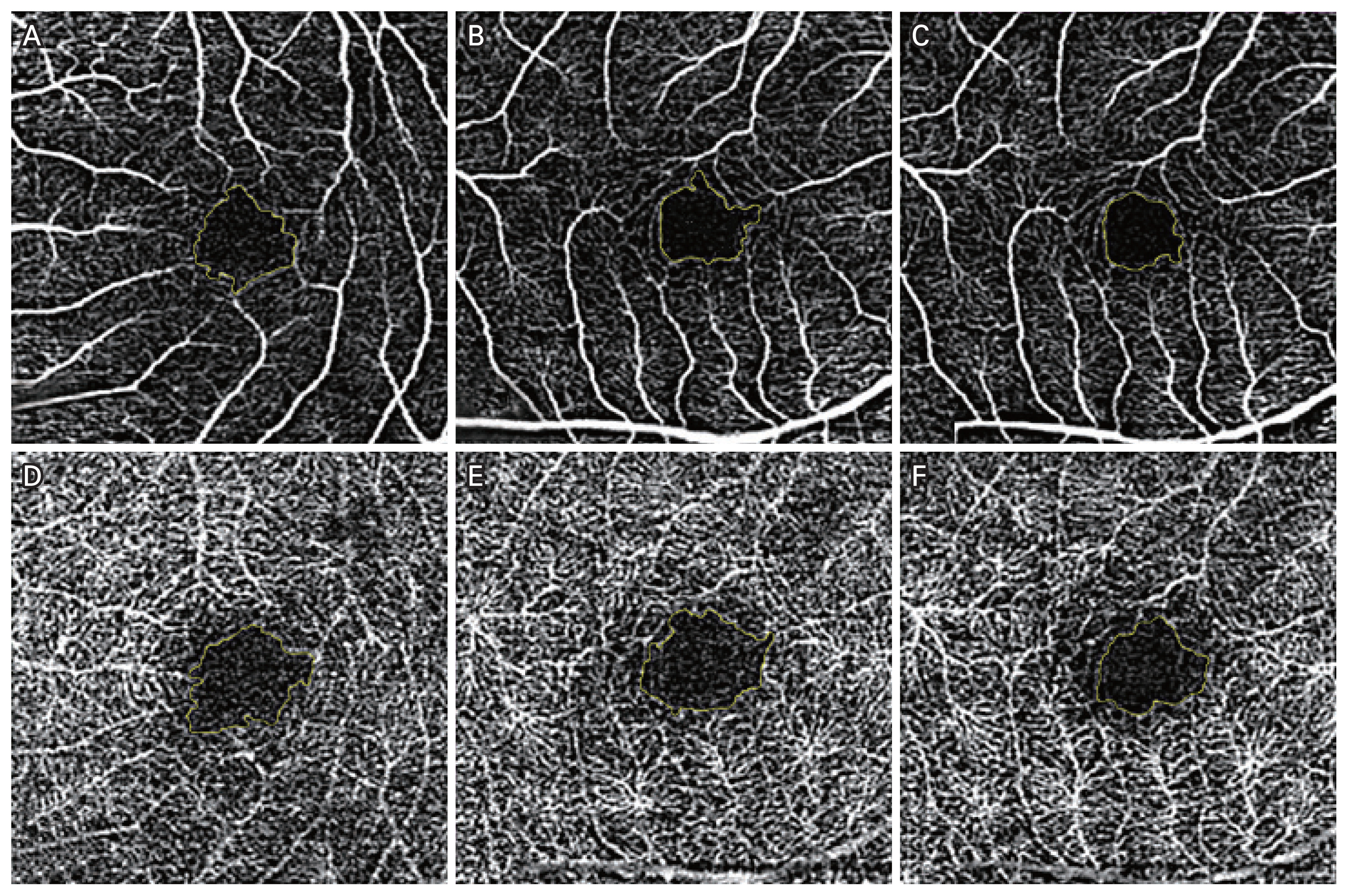
Fig. 2
The process of identifying participants with blunt ocular trauma (BOT). OCTA = optical coherence tomography angiography.

Fig. 3
The size of the foveal avascular zone (FAZ) area in (A) the deep capillary plexus and (B) the superficial capillary plexus. The mean values are indicated by the dots. The statistical analyses were performed with the Wilcoxon signed rank test and paired t-test. *p < 0.05.
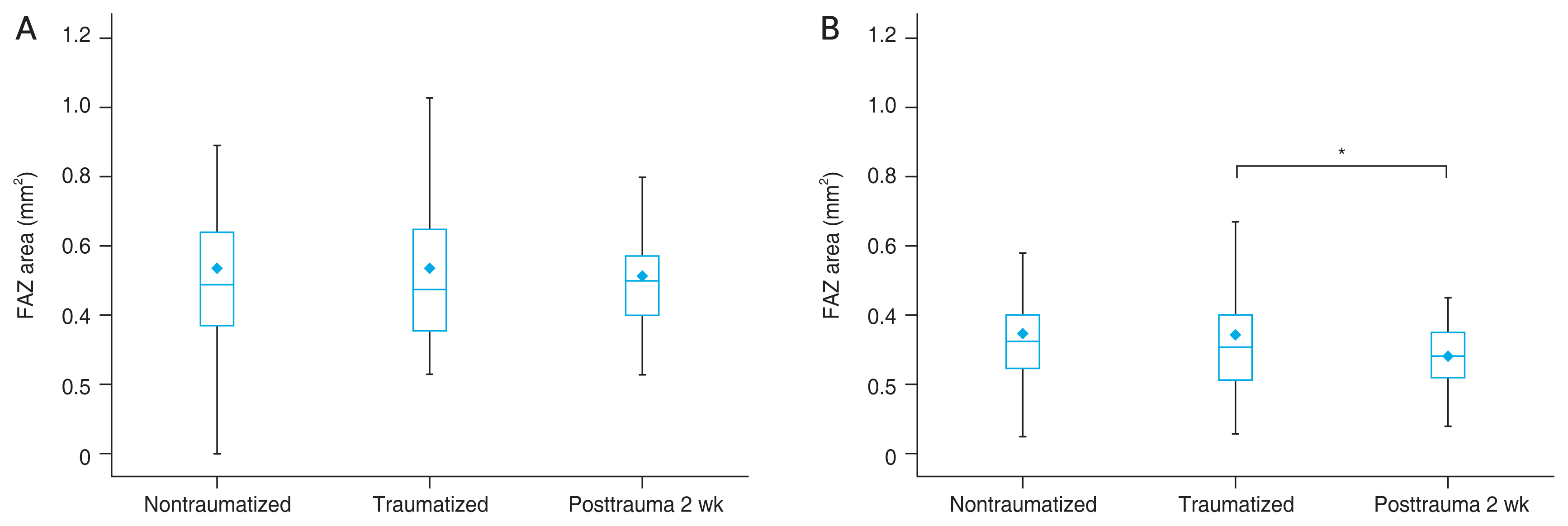
Table 1
Demographic features and optical coherence tomography angiography measurements of 48 blunt ocular trauma patients (96 eyes)
Table 2
The comparisons of mean foveal avascular zone area between nontraumatized eyes, traumatized eyes, and traumatized eyes 2 weeks after trauma
| Variable | Foveal avascular zone area (mm2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Deep capillary plexus | Superficial capillary plexus | |||||||||
|
|
|
|||||||||
| Nontraumatized | Traumatized | Posstrauma 2 wk | p-value | Nontraumatized | Traumatized | Posstrauma 2 wk | p-value | |||
|
|
|
|||||||||
| Nontraumatized vs. traumatized* | Traumatized vs. posstruama 2 wk† | Nontraumatized vs. traumatized* | Traumatized vs. posstruama 2 wk† | |||||||
| Total | 0.54 ± 0.20 | 0.54 ± 0.21 | 0.53 ± 0.18 | 0.96 | 0.20 | 0.34 ± 0.12 | 0.34 ± 0.13 | 0.30 ± 0.12 | 0.42 | 0.01 |
| BOF | ||||||||||
| Yes | 0.57 ± 0.24 | 0.58 ± 0.23 | 0.56 ± 0.19 | 0.76 | 0.33 | 0.34 ± 0.09 | 0.34 ± 0.11 | 0.35 ± 0.09 | 0.65 | 0.16 |
| No | 0.50 ± 0.16 | 0.48 ± 0.19 | 0.45 ± 0.13 | 0.66 | 0.37 | 0.34 ± 0.16 | 0.33 ± 0.13 | 0.29 ± 0.16 | 0.44 | 0.04 |



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print