 |
 |
| Korean J Ophthalmol > Volume 36(6); 2022 > Article |
|
Abstract
Purpose
To compare the diagnosis and treatment outcome of lacrimal drainage obstruction of patients who underwent systemic chemotherapy (CTx) or radioactive iodine treatment (RAI) by using dacryoendoscopy and at the same time performing dacryoendoscopy-guided silicone tube insertion (STI) to treat epiphora.
Methods
From July 2017 to December 2020, the medical records of 11 patients (16 eyes) were diagnosed with lacrimal drainage obstruction after CTx or RAI and underwent dacryoendoscopy-guided STI were reviewed retrospectively. We tried to count the number of obstructive sites in total patients using slit-lamp examination and dacryoendoscopic findings.
Results
A total of 11 patients, 16 eyes, were enrolled in this study. The onset of epiphora in the CTx group (3.0 ┬▒ 4.0 months) was significantly shorter than that in the RAI group (52.6 ┬▒ 36.5 months, p = 0.001). There were total 32 obstructive sites including 28 obstructive sites of dacryoendoscopic findings and four sites of punctual stenosis in total 16 cases. Using dacryoendoscopy, granulation findings was dominant in RAI patients (p = 0.038) and mucus finding was frequent mostly in lacrimal sac and canaliculus. In the CTx group, mucosal edema finding was dominant (p = 0.038) and fibrotic membrane finding was frequent in all levels of lacrimal drainage system. Regarding the obstructive location, lacrimal sac was the most frequently obstructed site in the two groups (p = 0.038).
Conclusions
The onset of epiphora in the CTx group was significantly earlier than in the RAI group. In the CTx group, mucosal edema finding was frequent in all levels of lacrimal drainage system. In the RAI group, granulation finding was frequent mostly in lacrimal sac and canaliculus. Since the clinical outcome was satisfactory, intervention with dacryoendoscopy-guided STI could be a treatment of choice in patients with epiphora after CTx or RAI.
Many studies have documented that systemic use of chemotherapy (CTx) agents can cause lacrimal drainage obstruction (LDO) and docetaxel is the most well-known drug causing LDO [1-6]. According to Mansur et al. [7], S-1 and radioactive iodine treatment (RAI) have been reported to cause a lacrimal duct failure. Noguchi et al. [8] also reported, the higher the concentration of docetaxel treatment, the more people complained of tear symptoms. Yet, the direct pathologic cause of this LDO has not been fully understood since we could not examine directly inside lacrimal drainage system (LDS) before application of dacryoendoscopy.
Currently, the primary surgical treatment for LDO is recanalization or bypass surgery for tear drainage with silicone tube insertion (STI). According to Kim et al. [9], LDO caused by S-1 responded well to STI in four cases. Here, we underwent dacryoendoscopy-guided STI in 11 patients, which can directly identify the location of the obstructive lesion inside LDS and recanalized the duct followed in order to improve the tearing symptom. The dacryoendoscopy has the advantage of being able to observe the LDS from the punctum to the Hasner valve in real time and check the lesions causing LDO. According to Lim et al. [10], it enabled them to visualize inside the lacrimal duct on site, as well as examine lesions that could not be identified in the dacryocystography (DCG).
In this study, we aimed to compare the dacryoendoscopic finding of LDO associated with systemic CTx or RAI for cancer treatment.
This study and data collection protocol were approved by the Institutional Review Board of CHA Bundang Medical Center, CHA University (No. 2017-02-003). Our study design adhered to the tenets of the Declaration of Helsinki. Informed consent about clinical information and specific consent about publication of the identifying information/images in an online open-access publication were obtained from each subject before enrollment.
From July 2017 to December 2020, the medical records of 312 patients diagnosed with LDO were reviewed retrospectively. A total of 11 patients, 16 eyes, were diagnosed with LDO and treated with dacryoendoscopy-guided STI after CTx and RAI. Case 1 to 8 individuals were the CTx group, whom with breast, ovarian, or lung cancer underwent CTx and the regimens were all different and multi-drug therapy. Since the dosage and component of the regimens vary, we did not classify according to the type of regimens. Case 9 to 16 individuals were the RAI group, whom with thyroid cancer underwent RAI (Table 1).
We carried out vision and intraocular pressure test, slit-lamp examination, history taking, and canaliculus irrigation test. Punctal stenosis was diagnosed under slit-lamp examination. The severity of epiphora was graded using Munk score. Tear meniscus height (TMH), punctal diameter, and punctal reserve were measured by optical coherence tomography (Spectralis OCT; Heidelberg Engineering, Heidelberg, Germany).
The irrigation test was performed by inserting a 26G needle with a blunt tip into a 2-mL syringe filled with normal saline solution, inserted into the punctum, and then injected saline to verify that it was passed over to the nose or throat. Results were divided by well passed and not passed.
Patients who were strongly suspected of LDO proceeded DCG. DCG was performed after instilled a drop of proparacaine 0.5% (Alcaine; Alcon, Fort Worth, TX, USA) into the conjunctival sac, check whether the contrast agent runs along the LDS by injecting the contrast agent, lohexol (Bonorex; Central Medical Service, Seoul, Korea) while scanning X-rays. By DCG, we classified primary pattern, such as narrowing or obstruction, and secondary pattern, such as beaded or secondary sac dilation.
By measurement of TMH, irrigation test, and DCG, patients were diagnosed with LDO. Patients who underwent multiple systemic CTx or RAI but did not know the exact drug were excluded. The cases that underwent additional ophthalmic surgery such as conjunctivochalasis, conjunctival lesion, or caruncle lesion were also excluded.
A single surgeon (HL) performed all operations. Surgical treatment was done under general or local anesthesia by using dacryoendoscopy and STI. After extending the punctum using the punctum dilator and spring scissor, by inserting the 0.9-mm diameter probe tip, bent type dacryoendoscope (Ruido Fiberscope; FiberTech Co., Tokyo, Japan) through the punctum, check the internal conditions of the LDS by flowing through saline, leading to the upper and lower canaliculus, lacrimal sac, lacrimal duct, and inferior meatus. The obstructive lesion was pushed out by the sheath, guided by the endoscopy and pressure of perfusion solution with a syringe connected with probe. A bicanalicular silicone tube with a diameter of 0.94 mm (Yoowon Meditec, Seoul, Korea) was inserted through the sheath under visual guidance. The sheath and the tube were retrieved, and both ends of the tube were locked and stabilized under the inferior meatus. The tube was removed after 6 months. Levofloxacin 0.5% (Cravit; Santen Pharmaceutical, Osaka, Japan) and fluorometholone 0.1% (Flumetholone, Santen Pharmaceutical) were used according to the clinical course after surgery.
Dacryoendoscopic findings were classified according to the location and features of the obstruction. It was classified into space-occupying and structural change according to the criteria that Lee and Lew [11] previously classified. Obstruction findings were observed in the LDS by dacryoendoscopy (Table 1). The space-occupying group included mucus, stones, and granulation findings which interfere with the flow of tears by secretion. The structural change group included fibrotic membrane, stenosis, and edematous findings that make mechanical resistance of the flow (Fig. 1A-1H).
We tried to count the number of obstructive sites in the total patients. Therefore, there were total 32 obstructive sites including 28 obstructive sites of dacryoendoscopic findings and four sites of punctual stenosis in 11 patients (Table 1).
Surgeries were deemed successful when the patientŌĆÖs subjective results are satisfied with result, lower TMH is less than 300 ╬╝m and the irrigation test was passed at 6 months after extubation.
Statistical analysis was performed using IBM SPSS ver. 27.0 (IBM Corp., Armonk, NY, USA). An independent t-test and Mann-Whitney U-test were used to compare parametric and nonparametric groups, respectively. A paired t-test was performed to compare before and after surgery data. Fisher exact test was used to determine a significant association between two treatment groups. We reported dichotomous outcomes as odds ratios and continuous outcomes as the mean and their respective 95 % confidence intervals. A p-value less than 0.05 was regarded as statistically significant.
A total of 11 patients, 16 eyes, were enrolled in this study. The mean age was 63.2 ┬▒ 11.8 years. The mean duration of the epiphora was 28.3 ┬▒ 35.5 months, and the onset of epiphora after treatment is 29.5 ┬▒ 36.5 months. The mean Munk score was 3.8 ┬▒ 1.6 and preoperative TMH was 458.1 ┬▒ 184.2 ╬╝m. The mean duration of tube insertion was 5.6 ┬▒ 0.6 months. The onset of epiphora (3.0 ┬▒ 4.0 months) in the CTx group was significantly shorter than that in the RAI group (52.6 ┬▒ 36.5 months, p = 0.001) (Table 2).
There was no difference between the two groups in the clinical outcomes of LDO patients. Well-passed findings were five (31.3%), and not passed were 11 (68.7%) in total. Primary pattern cases classified by DCG (narrowing, obstruction) were 11 (68.8%), and secondary pattern cases (beaded, secondary sac dilation) were five (31.3%). The success rate was 100% in both groups (Table 3).
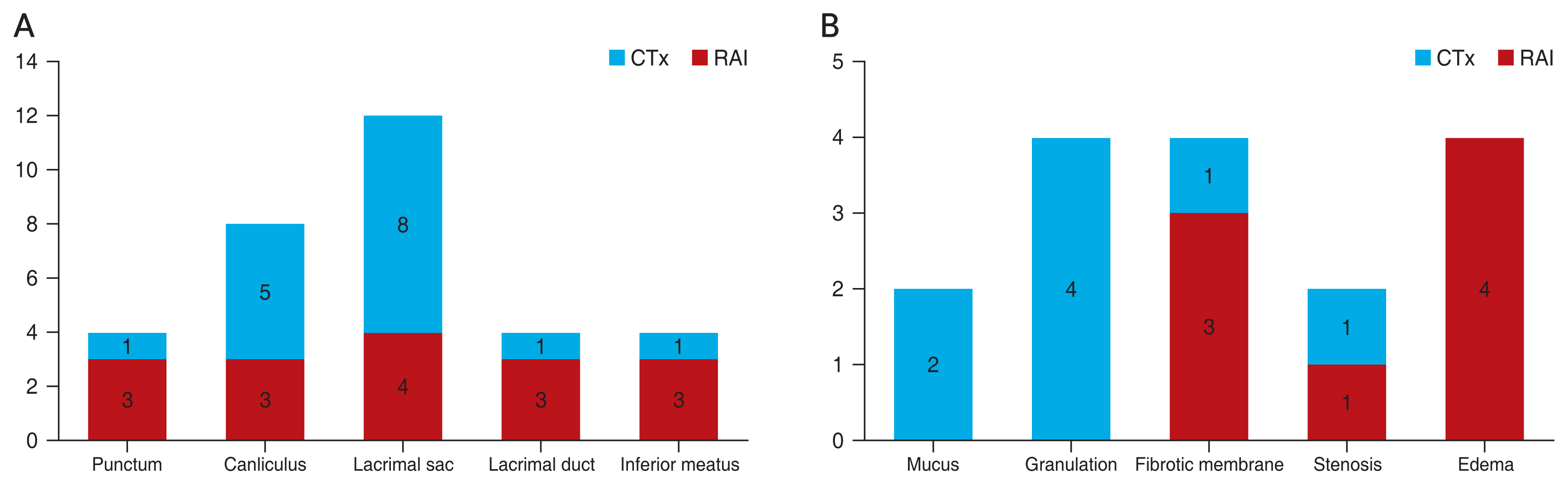
There were total 32 obstructive sites, including 28 obstructive sites of dacryoendoscopic findings and four sites of punctual stenosis in total 16 cases. Using dacryoendoscopy, granulation findings was dominant in RAI patients (p = 0.038) and mucus finding was frequent mostly in lacrimal sac and canaliculus. In the CTx group, mucosal edema finding was dominant (p = 0.038) and fibrotic membrane finding was frequent in all sites of LDS. Regarding the obstructive location, lacrimal sac was the most frequently obstructed site in both groups. However, there was no statistically significant difference in obstructed site except lacrimal sac (p = 0.038) (Fig. 2A, 2B and Table 3).
In this study, we reviewed the medical chart of patients who previously diagnosed LDO after systemic CTx or RAI and proceeded dacryoendoscopy-guided STI. Here, we aimed to compare the dacryoendoscopic finding of LDO related with systemic CTx or RAI using dacryoendoscopy.
The CTx groupŌĆÖs onset period of epiphora is significantly earlier than the RAI group. The mean onset period after CTx was 3.0 months, which is similar to 1.4-6.0 months as Park et al. [12] reported. Bartley [13] classified secondary acquired LDO by infectious, inflammatory, neoplastic, traumatic, or mechanical causes. CTx might cause systemic inflammation including LDS, so epiphora occurs immediately after CTx is also explainable.
In the CTx group, the frequency of obstructed site was similar in all the levels of LDS; four lacrimal sac (25.0) and three (18.8) each for punctum, canaliculus, lacrimal duct, and inferior meatus. In dacryoendoscopy, fibrotic membrane finding was frequent than RAI patients and edema finding was only found in CTx patients (Fig. 2B). Fibrotic membrane finding could be explained by the fact that the CTx regimens could affect by stabilizing microtubules halting cell division, causing keratinization and fibrotic change, which might be the reason of LDO after CTx [14]. Moreover, according to the biopsy-proven study by Esmaeli et al. [15] showed, the fibrotic changes in the lacrimal sac and the nasal mucosa showing marked keratinization after docetaxel. CTx agent can cause systemic inflammation and edema, causing upper part of LDO, which can explain the edema finding [16]. Fezza et al. [17] reported that CTx agent can make lacrimal sac and sinus mucosa to chronic inflammatory changes and Kim et al. [9] suggested that systemic inflammation effect of CTx agent can damage to LDS.
The mean onset after RAI (52.6 ┬▒ 36.5 months) was considered as delayed reaction than CTx (3.0 ┬▒ 4.0 months). Morgenstern et al. [18] described the relation between RAI and LDO due to the expression of a sodium iodine symporter system, which promotes iodide uptake, in the lacrimal sac and the lacrimal duct. Active iodine uptake mediated by sodium iodine symporter system could be responsible for damage to the lacrimal duct [19]. This could explain a mechanism that iodine slowly accumulated in lacrimal sac and cause a delayed reaction. It is a corresponding result with the dacryoendoscopic finding, the most obstructed site was lacrimal sac in RAI group (Fig. 2A) and has significant difference with the CTx group (p = 0.038) (Table 3). The most common findings of RAI as mucus and granulation could be explained regarding the delayed onset and related with the previously biopsy-proven foreign-body reaction performed by Shepler et al. [20]. There was an uptake at lacrimal sac by thyrogen scan after iodine treatment, biopsy results showed foreign-body reaction and fibrosis with no malignant cells. Iodine thought to be remained in the lacrimal sac after treatment and causes foreign-body reaction as mucus or granulation finding in dacryoendoscope [20]. Also I-131 therapy was reported to cause LDO, a relation likely to be dose-related [21].
In our cases, there were four cases with closed punctum during the slit-lamp examination. Punctal stenosis was observed in both CTx (n = 3) and RAI groups (n = 1). Eiseman et al. [22] reported 26.9% of ocular abnormality as tearing and 5.8% of them was punctal and canalicular stenosis after use of systemic 5-fluorouracil. Also, Kang et al. [23] confirmed the nasolacrimal duct was the most common obstruction site in patients who underwent radiotherapy (59%), whereas the punctum or canaliculus was mostly affected in patients treated with S-1 (94%) or docetaxel (100%). The patients treated with S-1 or docetaxel presented the punctum or canaliculus obstruction most commonly.
For both groups, the LDO might be a minor problem because they are getting through life-threatening treatment and underwent treatments that could affect their whole body. Also, LDO secondary to CTx and RAI is a newly recognized complication, which is not very well-known to majority of patients. However, physicians who manage patients proceeding CTx and RAI should be aware of this complication and sending patients to the ophthalmologist early for surgical intervention could improve the quality of life of patients and spare harder and larger surgery such as DCR.
There were several limitations to this study. First, the mean cumulative dose of systemic CTx and RAI received was uncertain. Further studies are needed to establish the relationship between cumulative dose and endoscopic treatment.
Second, the clinical findings inside the LDS were categorized according to the type and sites of the obstruction only prior to treatment. In this study, we were able to identify dacryoendoscopic findings predicting surgical outcomes. Further study on the follow up findings of the LDS after silicone tube removal will clarify our present findings related with the pathogenesis.
In conclusion, the onset of epiphora in the CTx group was significantly earlier. In the CTx group, mucosal edema finding was dominant and fibrotic membrane finding was frequent in all levels of LDS. In RAI group, granulation finding was dominant and mucus finding was frequent mostly in lacrimal sac and canaliculus. Since the clinical outcome was satisfactory, intervention with dacryoendoscopy-guided STI could be a treatment of choice in patients with epiphora after CTx or RAI. In addition, it enabled us to estimate the cause of LDO after anticancer therapy and know the characteristics of LDS for each anticancer treatment through dacryoendoscopy. The exact etiopathogenesis of LDO after chemotherapy is not yet known, but we hope this study will be helpful for further research.
Notes
Conflicts of Interest: Helen Lew is a member of the Editorial Board of the Korean Journal of Ophthalmology since 2015. However, she was not involved in the peer reviewer selection, evaluation, or decision process of this article. Otherwise, no other potential conflicts of interest relevant to this article were reported.
References
1. Esmaeli B, Valero V, Ahmadi MA, et al. Canalicular stenosis secondary to docetaxel (taxotere): a newly recognized side effect. Ophthalmology 2001;108:994-5.


2. Esmaeli B, Ahmadi MA, Rivera E, et al. Docetaxel secretion in tears: association with lacrimal drainage obstruction. Arch Ophthalmol 2002;120:1180-2.


3. Esmaeli B, Hortobagyi GN, Esteva FJ, et al. Canalicular stenosis secondary to weekly versus every-3-weeks docetaxel in patients with metastatic breast cancer. Ophthalmology 2002;109:1188-91.


4. Esmaeli B, Amin S, Valero V, et al. Prospective study of incidence and severity of epiphora and canalicular stenosis in patients with metastatic breast cancer receiving docetaxel. J Clin Oncol 2006;24:3619-22.


5. Leyssens B, Wildiers H, Lobelle JP, et al. A double-blind randomized phase II study on the efficacy of topical eye treatment in the prevention of docetaxel-induced dacryostenosis. Ann Oncol 2010;21:419-23.


7. Mansur C, Pfeiffer ML, Esmaeli B. Evaluation and management of chemotherapy-induced epiphora, punctal and canalicular stenosis, and nasolacrimal duct obstruction. Ophthalmic Plast Reconstr Surg 2017;33:9-12.


8. Noguchi Y, Kawashima Y, Kawara H, et al. A retrospective analysis of epiphora due to docetaxel. Gan To Kagaku Ryoho 2016;43:737-41.

9. Kim N, Park C, Park DJ, et al. Lacrimal drainage obstruction in gastric cancer patients receiving S-1 chemotherapy. Ann Oncol 2012;23:2065-71.


10. Lim SW, Sung YJ, Lew H. Clinical efficacy of lacrimal endoscopy in patients with epiphora. J Korean Ophthalmol Soc 2017;58:495-502.


11. Lee SM, Lew H. Transcanalicular endoscopic dacryoplasty in patients with primary acquired nasolacrimal duct obstruction. Graefes Arch Clin Exp Ophthalmol 2021;259:173-80.



12. Park J, Kim J, Baek S. Clinical features and treatment outcomes of patients with tearing after chemotherapy. Eye (Lond) 2019;33:746-53.




13. Bartley GB. Acquired lacrimal drainage obstruction: an etiologic classification system, case reports, and a review of the literature. Part 1. Ophthalmic Plast Reconstr Surg 1992;8:237-42.

14. McCartney E, Valluri S, Rushing D, et al. Upper and lower system nasolacrimal duct stenosis secondary to paclitaxel. Ophthalmic Plast Reconstr Surg 2007;23:170-1.


15. Esmaeli B, Burnstine MA, Ahmadi MA, et al. Docetaxel-induced histologic changes in the lacrimal sac and the nasal mucosa. Ophthalmic Plast Reconstr Surg 2003;19:305-8.


16. Vyas D, Laput G, Vyas AK. Chemotherapy-enhanced inflammation may lead to the failure of therapy and metastasis. Onco Targets Ther 2014;7:1015-23.



17. Fezza JP, Wesley RE, Klippenstein KA. The treatment of punctal and canalicular stenosis in patients on systemic 5-FU. Ophthalmic Surg Lasers 1999;30:105-8.


18. Morgenstern KE, Vadysirisack DD, Zhang Z, et al. Expression of sodium iodide symporter in the lacrimal drainage system: implication for the mechanism underlying nasolacrimal duct obstruction in I(131)-treated patients. Ophthalmic Plast Reconstr Surg 2005;21:337-44.


19. Cetinkaya A, Kersten RC. Relationship between radioactive iodine therapy for thyroid carcinoma and nasolacrimal drainage system obstruction. Ophthalmic Plast Reconstr Surg 2007;23:496.

20. Shepler TR, Sherman SI, Faustina MM, et al. Nasolacrimal duct obstruction associated with radioactive iodine therapy for thyroid carcinoma. Ophthalmic Plast Reconstr Surg 2003;19:479-81.


21. Kloos RT, Duvuuri V, Jhiang SM, et al. Nasolacrimal drainage system obstruction from radioactive iodine therapy for thyroid carcinoma. J Clin Endocrinol Metab 2002;87:5817-20.


Fig.┬Ā1
Dacryoendoscopic findings after chemotherapy (CTx) and radioactive iodine treatment (RAI). (A) Case 2, mucosal edema after CTx (docetaxel, carboplatin, trastuzumab, pertuzumab) in the canaliculus. (B) Case 5, mucosal edema after CTx (pemetrexed, carboplatin) in the lacrimal sac. (C) Case 2, mucosal edema after CTx (docetaxel, carboplatin, trastuzumab, pertuzumab) in the lacrimal duct. (D) Case 8, membranous stenosis after CTx (ifosfamide, methotrexate, etoposide) in the inferior meatus. (E) Case 16, mucosal granulation (asterisk) after RAI in the canaliculus. (F) Case 13, mucosal granulation (asterisk) after RAI in the lacrimal sac. Case 12, (G) mucosal granulation (asterisk) after RAI in the lacrimal duct and (H) mucosal edema after RAI in the inferior meatus.
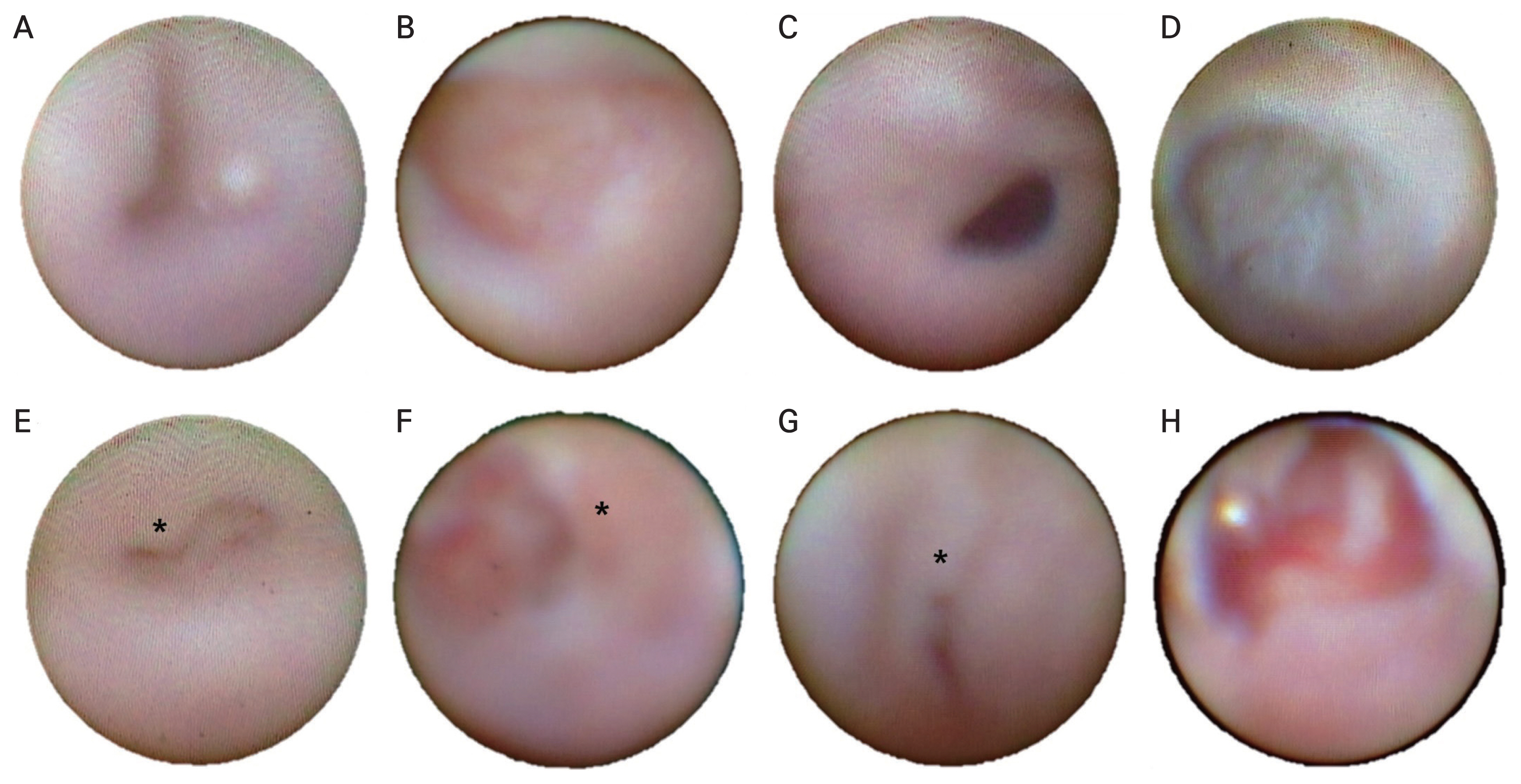
Fig.┬Ā2
Dacryoendoscopic findings in the chemotherapy (CTx) and radioactive iodine treatment (RAI) groups. (A) Obstructive location. (B) Obstructive features.
Table┬Ā1
Demographics of lacrimal drainage obstruction patients with CTx and RAI
CTx = chemotherapy; RAI = radioactive iodine treatment; Preop = preoperative; TMH = tear meniscus height; Postop = postoperative; U = upper punctum; L = lower punctum; DCG = dacryocystography; SP = status post; LD = lacrimal duct; C = canaliculus; LS = lacrimal sac; CCO = common canalicular obstruction; IM = inferior meatus; NDO = nasolacrimal drainage obstruction; NK/T = natural killer/t-cell; Tx = treatment.
Table┬Ā2
Clinical characteristics of CTx and RAI groups treated with dacryoendoscopy-guided silicone tube insertion
| Characteristic | CTx (n = 8) | RAI (n = 8) | Total (n = 16) | p-value |
|---|---|---|---|---|
| Age (yr) | 69.0 ┬▒ 8.2 | 56.2 ┬▒ 12.3 | 63.2 ┬▒ 11.8 | 0.065 |
| Sex | 0.394 | |||
| ŌĆāMale | 2 | 8 | 10 | |
| ŌĆāFemale | 6 | 0 | 6 | |
| Epiphora onset after treatment (mon) | 3.0 ┬▒ 4.0 | 52.6 ┬▒ 36.5 | 29.5 ┬▒ 36.5 | 0.001* |
| Epiphora duration (mon) | 60.5 ┬▒ 64.3 | 20.3 ┬▒ 11.5 | 28.3 ┬▒ 35.5 | 0.370 |
| Munk score | 4.1 ┬▒ 1.8 | 3.4 ┬▒ 1.4 | 3.8 ┬▒ 1.6 | 0.277 |
| Tear meniscus height (╬╝m) | 478.3 ┬▒ 180.0 | 435.0 ┬▒ 209.8 | 458.1 ┬▒ 184.2 | 0.815 |
| Duration of tube insertion (mon) | 5.7 ┬▒ 0.9 | 5.5 ┬▒ 0.6 | 5.6 ┬▒ 0.6 | 0.667 |
Table┬Ā3
Clinical outcomes associated with dacryoendoscopic findings from CTx and RAI groups treated with dacryoendoscopy-guided silicone tube insertion
| Clinical outcome | CTx | RAI | Total | p-value |
|---|---|---|---|---|
| Irrigation test (no. of cases) | ||||
| ŌĆāWell passed | 2 (25.0) | 3 (37.5) | 5 (31.3) | 0.395 |
| ŌĆāNot passed | 6 (75.0) | 5 (62.5) | 11 (68.7) | 0.968 |
| DCG finding (no. of cases) | ||||
| ŌĆāPrimary | 6 (75.0) | 5 (62.5) | 11 (68.7) | >0.999 |
| ŌĆāSecondary | 2 (25.0) | 3 (37.5) | 5 (31.3) | >0.999 |
| Success rate (no. of cases) | 8 (100) | 8 (100) | 16 (100) | >0.999 |
| Obstructive location (no. of sites) | ||||
| ŌĆāPunctum | 3 (18.8) | 1 (6.3) | 4 (12.5) | 0.285 |
| ŌĆāŌĆāCanaliculus | 3 (18.8) | 5 (31.3) | 8 (25.0) | 0.310 |
| ŌĆāŌĆāLacrimal sac | 4 (25.0) | 8 (50.0) | 12 (37.5) | 0.038* |
| ŌĆāŌĆāLacrimal duct | 3 (18.8) | 1 (6.3) | 4 (12.5) | 0.285 |
| ŌĆāŌĆāInferior turbinate | 3 18.8) | 1 (6.3) | 4 (12.5) | 0.285 |
| ŌĆāTotal | 16 (100) | 16 (100) | 32 (100) | |
| Obstruction feature (no. of sites) | ||||
| ŌĆāSpace-occupying | ||||
| ŌĆāŌĆāMucus | 0 (0) | 4 (25.0) | 4 (12.5) | 0.233 |
| ŌĆāŌĆāGranulation | 0 (0) | 7 (43.8) | 7 (21.9) | 0.038* |
| ŌĆāStructural change | ||||
| ŌĆāŌĆāFibrotic membrane | 6 (37.5) | 2 (12.5) | 8 (25.0) | 0.285 |
| ŌĆāŌĆāStenosis | 4 (25.0) | 3 (18.8) | 7 (21.9) | 0.767 |
| ŌĆāŌĆāEdema | 6 (37.5) | 0 (0) | 6 (18.8) | 0.038* |
| ŌĆāTotal | 16 (100) | 16 (100) | 32 (100) | |



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




