Comparison of Anterior Segment Measurements with a New Multifunctional Unit and Five Other Devices
Article information
Abstract
Purpose
To evaluate the clinical availability of a multifunctional ocular biometric unit, MR-6000, for simultaneous keratometry, tonometry, topography, and pachymetry evaluation, and compare anterior segment measurements with five other devices: autokeratometer (KR-1), Scheimpflug camera (Pentacam HR), swept-source optical coherence tomography (IOLMaster 700), Placido disk scanning-slit topography (Orbscan II), and noncontact tonometry (FT-1000).
Methods
Thirty eyes from thirty patients who visited Severance Hospital for cataract surgery were examined using MR-6000 and the other devices. The mean keratometry, central corneal thickness (CCT), white-to-white (WTW) distance, and intraocular pressure (IOP) values were compared. Repeated measures analysis of variance, Wilcoxon signed-rank test, intraclass correlation coefficient (ICC), and Bland-Altman plot were used to assess the correlation and agreement between devices.
Results
Thirty eyes of thirty patients were evaluated. Statistically significant differences in mean keratometry between MR-6000, KR-1, Pentacam HR, and IOLMaster 700 were not observed (p > 0.05). All five devices, including Orbscan II, had almost perfect agreement in measuring keratometry (ICC > 0.80, p < 0.05). CCT measured by MR-6000 was significantly different from that of Pentacam HR and Orbscan II measurements (p < 0.05) but correlated with that of Pentacam HR and Orbscan II measurements (ICC > 0.60, p < 0.05). The WTW distance measured by MR-6000 was not significantly different from that measured by IOLMaster 700 but was different from that measured by Orbscan II. IOP measured by MR-6000 was not correlated with FT-1000.
Conclusions
Keratometric values obtained through MR-6000 can be used interchangeably with other devices based on good correlation and agreement. However, the CCT, WTW, and IOP values were not interchangeable with a single multifunctional unit for cataract surgery preoperative examination.
Precise measurements of the ocular anterior segment, including corneal refractive power, corneal thickness, and anterior chamber depth, are important factors for cataract and corneal refractive surgery, as well as postoperative evaluation. In particular, accurate measurement of corneal refractive power determines the quality of postoperative visual acuity and is an indicator of residual refractive error. Corneal thickness is an important factor in deciding the surgical method when planning phakic intraocular lens implantation and laser in situ keratomileusis. In the new era of intraocular lens techniques and the advent of laser refractive surgery, accurate measurements of anterior ocular biometry and considering them together are becoming much more important. In many previous studies, repeatability and accordance with preexisting devices measuring the anterior segment have been proven; however, whether measurements from various devices are interchangeable remains controversial [1–4].
To measure anterior ocular biometry, Placido disk reflection, slit-scanning tomography, a rotating Scheimpflug camera, and anterior segmental optical coherence tomography are mainly used. Orbscan II (Bausch and Lomb, Rochester, NY, USA) adopted a Placido disk and scanning-slit topography system. With the Placido disk, assessment of the corneal surface curvature is possible, and the scanning-slit analyzes the posterior surface; hence, calculating corneal thickness is also possible [5]. Pentacam HR (Oculus, Wetzlar, Germany), using the rotating Scheimpflug camera analysis system, can evaluate the entire ocular anterior segment from the anterior corneal surface to the posterior lens surface. With Scheimpflug tomography and three-dimensional chamber analysis, anterior chamber depth, pachymetry, and keratometry measurements are possible [6,7]. When simply measuring corneal refractive power, auto kerato-refractometers are generally used, and one of them, KR-1 (Topcon, Tokyo, Japan), measures corneal refractive power using a mirror image of the eccentric rotation of the measurement ring in a 3-mm-diameter zone [8]. However, preexisting auto kerato-refractometers cannot measure ocular biometry other than simple corneal refraction; therefore, measurements using other devices, as described above, should be considered together for the preoperative measurement of cataract and refractive surgeries.
Previously, to measure the axial length for intraocular lens calculation before cataract surgery, an applanation A-scan ultrasound (US), usually a 10-MHz acoustic wave transducer, was the standard [9]. However, due to corneal damage as a contact biometry, inconsistent results due to corneal indentation, and practitioner error, the development of an optical biometer, IOLMaster (Carl Zeiss, Oberkochen, Germany), has replaced A-scan US as the preoperative biometry measurement for cataract surgery [10,11]. IOLMaster 700 uses swept-source optical coherence tomography with 1,050-mm laser infrared light, in six scan lines at 0°, 30°, 60°, 90°, 120°, and 150° to measure the axial length, anterior chamber depth, and central corneal thickness (CCT). With 18 reference points in the hexagonal pattern at 1.5-, 2.4-, and 3.2-mm optical zones, the corneal curvature can be measured [12,13].
The recently developed MR-6000 (Tomey Corp., Nagoya, Japan) is a multifunctional anterior ocular biometric device that can measure six different eye parameters in 90 seconds, including refraction, keratometry, topography, tonometry, pachymetry, and dry eye measurements. The MR-6000 is equipped with automatic alignment of three measurements cones, and the fast exchange of measurement cones in less than 4 seconds is possible. Also, before the measurement, the examiner can activate each measurement mode selectively. Regarding refraction and keratometry, using the same method as the auto kerato-refractometer, MR-6000 assumes that the cornea is a convex mirror, and an automated keratometer instantly records the size and computes the radius of the curvature while focusing the reflected corneal image onto an electronic photosensitive device. With this method, the flat and steep meridians (keratometric values) on the 3.0-mm diameter central cornea can be acquired [14]. The relatively quick refraction mode allows measurement of refraction values within seconds, even in situations of fixation loss in the case of uncooperative patients and children. In tonometry and pachymetry, with a new generation of air-cut function, the amount of air injection is reduced, more accurate measurements are enabled, and automated IOP correction is possible by reflecting pachymetry values. In topography, using 16 mire rings, an 8-mm diameter corneal area is examined, and several map types lead to a wide range of corneal shapes displaying opportunities. The Kerato-Asymmetry Index, Kerato-Regularity Index, spherical equivalent, pupil size, and white-to-white (WTW) distance can also be measured.
With the continuous development of anterior segment-measuring devices, it has become difficult to standardize the series of cataract preoperative examinations. As an ophthalmologist, it is difficult to discern which device measurement is the most reliable and necessary. In this situation, the release of a multifunctional ocular anterior biometric device may save time and cost for both patients and doctors. This study aimed to compare the measurements of the anterior segment of eyes with cataracts through MR-6000 and other conventional devices (KR-1, Pentacam HR, IOLMaster 700, Orbscan II), and tonometry (FT-1000; Tomey Corp., Tokyo, Japan) before cataract surgery, and evaluate their clinical utility.
Materials and Methods
Ethics statement
This retrospective study was based on a systematic review of the medical records of patients planning cataract surgery, approved by the Institutional Review Board of Severance Hospital (No. 2021-2460-001), and conducted in accordance with the Declaration of Helsinki. The written informed consent for publication of the research details was exempted due to the retrospective study design.
Study participants and measurement process
The study included 30 eyes from 30 patients who planned to undergo cataract surgery at Severance Hospital and underwent preoperative examination of the anterior segment using six devices (MR-6000, KR-1, Pentacam HR, IOLMaster 700, Orbscan II, and FT-1000) on the same day by a single technician at a single center between January 2021 and February 2021. Patients who had ocular conditions, such as keratoconjunctival diseases, that could affect the results; a history of intraocular or corneal surgery, such as laser corneal refractive surgery or ocular trauma; and those who used contact lenses within 1 week were excluded. A series of measurements were performed on the same day by the same skilled technician. Sequentially, using KR-1, FT-1000, MR-6000, Pentacam HR, IOLMaster 700, and Orbscan II, anterior segmental examinations were performed, and measurements were used for statistical analysis. Before examination with each device, the tear film was made uniform by blinking, and the patients were instructed to not move or blink during the examination. The technician carried out the examinations with care not to press the eyeball. Approximately 5 minutes was needed to move between the devices.
Measured parameters and data collection
Demographic information included age, sex, and, among the multiple device measurements, corneal keratometry, steep keratometry (Ks), and flat keratometry (Kf) of the anterior curvature. Corneal power (mean keratometry [Km]) for the flat and steep meridians was calculated through averaging: (Kf + Ks) / 2. Additionally, the CCT, WTW distance, and intraocular pressure (IOP) measured by each device were collected and compared.
Keratometry measured in diopters was calculated using the corneal refractive index (1.3375) in all devices (MR-6000, KR-1, Pentacam HR, IOLMaster 700, and Orbscan II). MR-6000 could choose the radius of the corneal anterior surface curvature from a central area (3.0 or 2.5 mm), which was set at a 3.0-mm radius area for measurement. KR-1, Pentacam HR, and Orbscan II measured a 3.0-mm radius from the corneal center. Keratometric values in the IOLMaster 700 were calculated using six light spots in a 2.5-mm radius of the corneal center.
The CCT value was used in the analysis center of the pachymetry map in MR-6000 and Orbscan II, and for analyzing the corneal thickness at the apex in Pentacam HR. The WTW distance was measured as the horizontal distance between the border of the corneal limbus using the topography system in both MR-6000 and Orbscan II. IOP was measured using noncontact tonometry (NCT) in both MR-6000 and FT-1000. The IOP value measured by the MR-6000 was collected as the CCT-corrected IOP using its own IOP correction formula: Δpressure = (554 − CCT) × 0.045.
Statistical analysis
Statistical analyses were performed using the IBM SPSS ver. 25.0 (IBM Corp., Armonk, NY, USA). The normal distribution was evaluated using the Kolmogorov-Smirnov test, and all measurements were tested for normality. All statistical values are presented as the mean ± standard deviation. In the paired comparison, a paired t-test was first considered, and the Wilcoxon signed-rank test was used if samples were not normally distributed. When comparing more than three devices, repeated measures analysis of variance (ANOVA) was used to compare measurements between devices, and Bonferroni test was used for post hoc analysis. Agreement between different devices was evaluated using intraclass correlation coefficients (ICCs) with 95% limits of agreement (LoAs; calculated as mean difference ± 1.96 × standard deviation of the difference) and a Bland-Altman plot. Interdevice absolute agreement of each measurement was assessed by calculating the ICCs from a two-way random effect, single measurement model: ICC (2, 1). According to the method suggested by Landis and Koch [15], the strength of agreement was categorized as follows: 0 as poor, 0 to 0.20 as slight, 0.21 to 0.40 as fair, 0.41 to 0.60 as moderate, 0.61 to 0.80 as substantial, and 0.81 to 1.00 as almost perfect. A p-value less than 0.05 were considered statistically significant.
Results
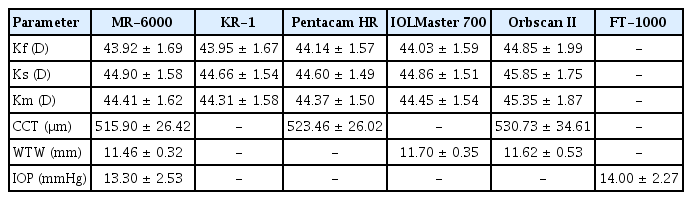
Thirty eyes of 30 patients (12 male and 18 female patients) scheduled for cataract surgery were evaluated. Their mean age was 66.52 ± 12.20 years (range, 38 to 83 years). Of the 30 eyes, 15 were the left eye and the other 15 were the right eye. The Km measured by the MR-6000, KR-1, Pentacam HR, IOLMaster 700, and Orbscan II was 44.41 ± 1.62, 44.31 ± 1.58, 44.37 ± 1.50, 44.45 ± 1.54, and 45.35 ± 1.87 diopters (D), respectively. The CCT was measured using the MR-6000, Pentacam HR, and Orbscan II. The mean CCT value was 515.0 ± 26.42, 523.46 ± 26.02, and 530.73 ± 34.61 μm, respectively. The WTW distance using the three devices MR-6000, IOLMaster 700, and Orbscan II was 11.46 ± 0.32, 11.70 ± 0.35, and 11.62 ± 0.53 mm, respectively. The mean IOP measured by the MR-6000 and FT-1000 was 13.30 ± 2.53 and 14.00 ± 2.27 mmHg, respectively (Table 1).
When comparing the Km among the five devices by repeated measures ANOVA, no statistically significant differences were found in the measurements of the four devices (MR-6000, KR-1, Pentacam HR, and IOLMaster 700; p > 0.05), except for KR-1 and IOLMaster 700 (p = 0.03). In particular, MR-6000 showed the smallest mean difference in comparison with Pentacam HR and IOLMaster 700 (0.03 and −0.04 D, respectively). However, comparing Orbscan II with that of the four other devices, p-values were below 0.001, and the mean differences with Orbscan II were greater than −0.7 D. We found that Orbscan II tended to measure keratometric values significantly higher than that with the other devices (Table 2). The mean differences between MR-6000 and each device ranged from −1.00 to 0.11 D. Almost all values were within the 95% LoA (Fig. 1A–1D).
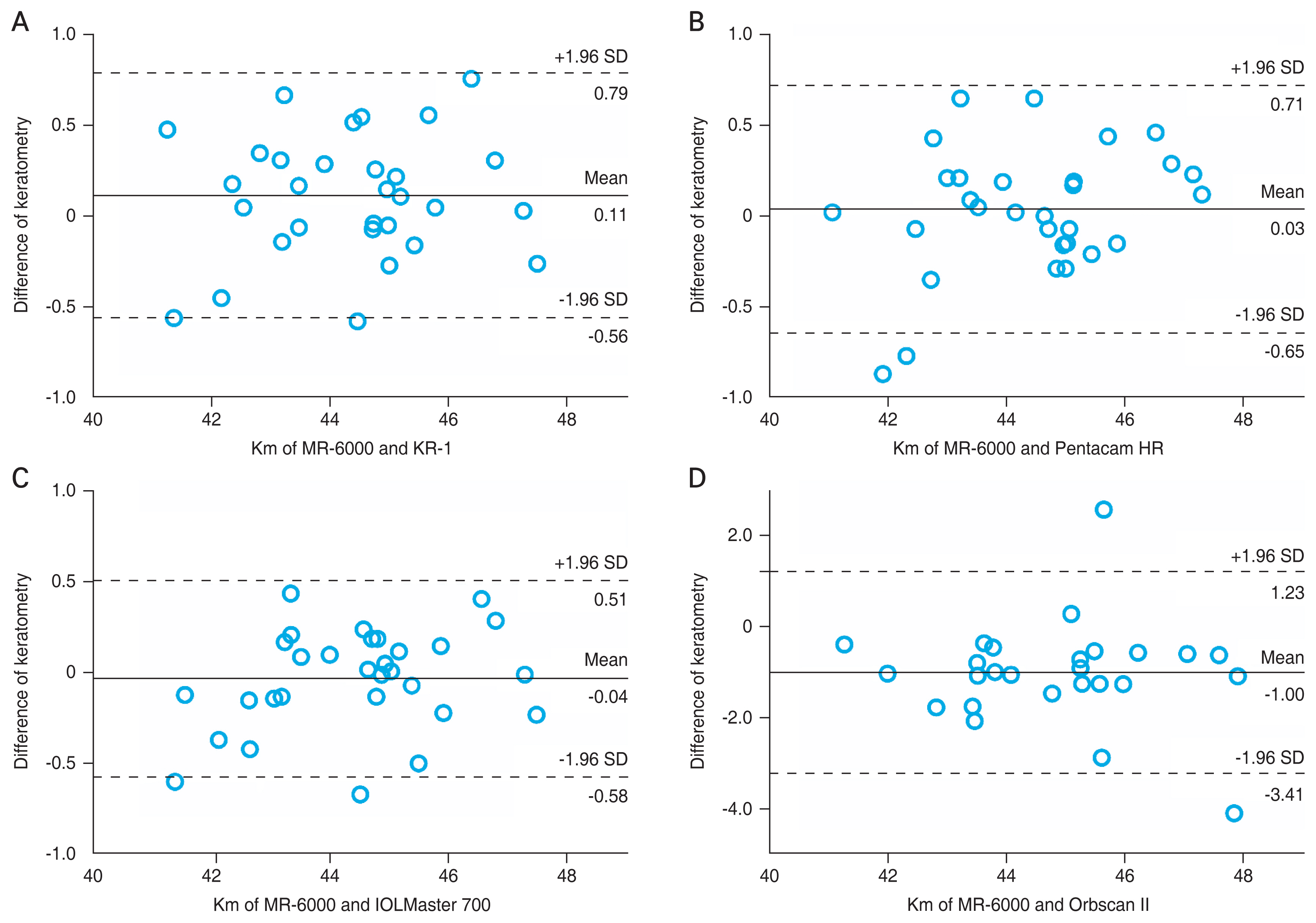
Agreement of mean keratometry (Km) measurement between MR-6000 (Tomey Corp., Nagoya, Japan) and other devices through the Bland-Altman plot. The solid line indicates the mean difference, and the dotted line shows the 95% limits of agreement. Bland-Altman plots that show agreements between MR-6000 and (A) KR-1 (Topcon, Tokyo, Japan), (B) Pentacam HR (Oculus, Wetzlar, Germany), (C) IOLMaster 700 (Carl Zeiss, Oberkochen, Germany), and (D) Orbscan II (Bausch and Lomb, Rochester, NY, USA). SD = standard deviation.
A high correlation was observed between the five keratometry devices. All ICCs were above 0.95, except when compared with that of Orbscan II (ICC value range, 0.844 to 0.913) (Table 3). Consistent with the result of the repeated measures ANOVA, the ICC showed an almost perfect correlation and agreement between devices (all ICC > 0.80, almost perfect) according to Landis and Koch’s method [15].

Correlation and agreement of mean keratometric values among MR-6000 and four other devices through ICC
The mean difference in the CCT between MR-6000 and Pentacam HR was −7.53 μm at 95% LoA (−11.816 to −3.250). When comparing MR-6000 with that of Orbscan II, the mean difference was −14.80 μm at 95% LoA (−25.046 to −4.554), and significant differences in the CCT were observed between MR-6000 and Pentacam HR and between MR-6000 and Orbscan II (p = 0.001 and 0.006, respectively). MR-6000 tended to measure CCT smaller than that with the other two devices. Meanwhile, a significant difference in the CCT was not found between Pentacam HR and Orbscan II (p = 0.124), with a mean difference of −7.27 μm, the smallest difference among the three devices. Comparison of the WTW distance measured by MR-6000 was significantly different from that measured by Orbscan II (p = 0.026). However, significant differences were not found between MR-6000 and Orbscan II and between IOLMaster 700 and Orbscan II ( p = 0.050 and 0.503, respectively). The IOP measurements did not satisfy the normality distribution, and Wilcoxon signed-rank test was performed. A significant difference between MR-6000 and FT-1000 was not noted (p = 0.180) (Table 4).

Comparison of central corneal thickness, white-to-white, and intraocular pressure values among devices through repeated measures ANOVA and Wilcoxon signed-rank test
When analyzing the correlation and agreement of CCT, WTW distance, and IOP through the ICC (2, 1), CCT measurements by MR-6000 showed substantial agreement with that of Pentacam HR and Orbscan II measurements (MR-6000 vs. Pentacam HR, ICC = 0.934; MR-6000 vs. Orbscan II, ICC = 0.719; Pentacam HR vs. Orbscan II, ICC = 0.798). When measuring the WTW distance, MR-6000 and IOLMaster 700 showed good agreement (ICC = 0.812, p < 0.001), but agreements between MR-6000 and Orbscan II and between IOLMaster 700 and Orbscan II were not statistically significant (p = 0.688 and 0.395, respectively). A significant correlation between the IOP measured by MR-6000 and FT-1000 was not observed (ICC = 0.317, p = 0.155) (Table 5). Significant agreements between MR-6000 and other devices in CCT and WTW distance measurements were analyzed and expressed through Bland-Altman plots (Fig. 2A–2C).
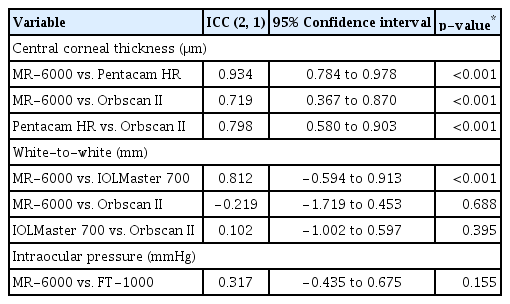
Correlation and agreement of central corneal thickness, white-to-white, and intraocular pressure by ICC
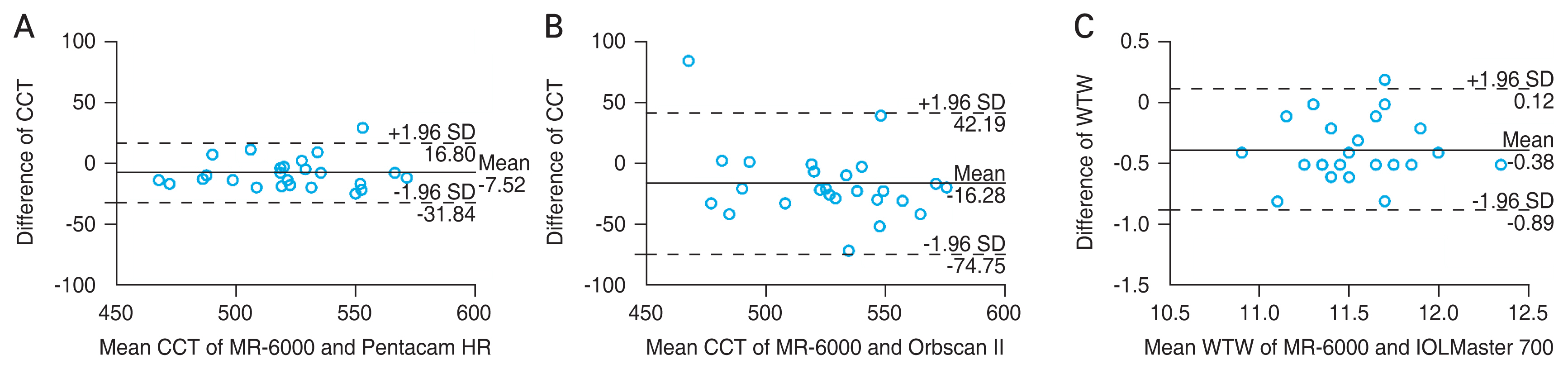
Agreement of central corneal thickness (CCT) and white-to-white (WTW) distance measurements between MR-6000 (Tomey Corp., Nagoya, Japan) and other devices through the Bland-Altman plot. The solid line indicates the mean difference, and the dotted line shows the 95% limits of agreement. Bland-Altman plot that show agreement of (A) CCT measurement between MR-6000 and Pentacam HR (Oculus, Wetzlar, Germany), (B) CCT measurement between MR-6000 and Orbscan II (Bausch and Lomb, Rochester, NY, USA), and (C) WTW distance between MR-6000 and IOLMaster 700 (Carl Zeiss, Oberkochen, Germany). SD = standard deviation.
Discussion
Accurate measurement of the anterior segments of the eye is important for predicting the results before cataract and refractive surgery and for postoperative management. Factors that accurately predict refractive power after cataract surgery include correct calculation of anterior segment parameters, qualitative management of the intraocular lens, and surgeons’ techniques [16,17]. Among these factors, errors that may occur during biometric measurements can significantly influence refractive error after surgery. Inaccurate measurement of corneal refractive power is one of the main causes of intraocular lens calculation errors [18]. According to Jo et al. [19], a 0.5 D error in corneal refractive power can yield a ±1.17 D difference in intraocular lens calculation. Recently, anterior ocular biometrics measured by various devices, such as auto kerato-refractometer, videokeratoscopy, and partial coherence interferometry, have been reported to be accurate and reproducible, but the measured values are known to be slightly different across devices. Therefore, considering the rapid development of new devices, the evaluation of their accuracy, reproducibility, and agreement with existing devices is very important.
In this study, using MR-6000, a novel multifunctional unit examining anterior segments through the combination of topography, autokeratometry, tonometry, pachymetry, and dry eye evaluation, we investigated whether MR-6000 could replace existing devices in measuring keratometry, CCT, WTW distance, and IOP. In the current circumstance, anterior biometric measurements are performed separately on each device, wasting time, space, and human resources for each examination. Eventually, time extension through a series of examinations leads to complaints regarding medical services, resulting in poor medical quality and low patient satisfaction. In outpatient care settings, the phenomenon of a patient waiting is perceived more negatively than that in general waiting conditions [20]; therefore, reducing the waiting time can lead to meeting patient satisfaction and improving medical quality [21]. In this respect, if the accuracy and consistency of measurements with the MR-6000 are significant compared to that of existing devices, the use of multiple devices can be replaced with a single device, saving time during examinations, and elevating the quality of medical services.
The results showed that the Km measured by the MR-6000 was not statistically different from the Km measured by the KR-1, Pentacam HR, and IOLMaster 700. MR-6000 had an almost perfect agreement with KR-1, Pentacam HR, IOLMaster 700, and Orbscan II in measuring Km. However, a relatively weak agreement was observed when compared to that of Orbscan II. Orbscan II tends to measure a larger keratometric value than that with the other devices. Consistent with our study, according to Han et al. [22], comparing keratometry measured using an auto kera-to-refractometer (KR-7100; Topcon), IOLMaster, and Orbscan II, Km was found to be greater with Orbscan II than that with IOLMaster or an autokeratometer. Whang et al. [23] compared keratometric values obtained using a manual keratometer (OM-4; Topcon), autokeratometer (RK-5; Canon, Tokyo, Japan), Orbscan II (Bausch and Lomb Surgical, Munich, Germany), IOLMaster (Carl Zeiss, Jena, Germany), and Pentacam HR (Oculus, Dudenhofen, Germany). Among them, the mean keratometric values measured by Orbscan II showed a greater mean absolute error. In another previous study, Kim and Chung [24] reported that the corneal curvature difference was less than 0.82 D, when compared with that of an autorefractor (RKF1, Canon), IOLMaster, and Orbscan II, and repeatability values did not significantly differ among devices. In our study, significant differences in Km among devices were not found except with Orbscan II, and intraclass correlation and the Bland-Altman plot showed great agreement among the devices, including Orbscan II. The tendency of Orbscan II to measure greater corneal refractive power than that with the others was similarly observed in previous studies [25–27]. The consistent measurement of corneal curvature with Orbscan II is probably due to the different measuring systems used in the Placido disk scanning-slit system and the relatively longer measuring time of Orbscan II. The acquisition time of Orbscan II is longer than that of the other devices, which prevented patients from blinking and may result in an unstable tear film and false corneal irregularity [28].
The CCT measured by Pentacam HR tended to be thicker than that measured by Orbscan II in previous studies [29–31]. Meanwhile, some studies have reported that the CCT was thicker when measured with Orbscan II than that with Pentacam HR [32,33]. In this study, corneal thickness was as follows, in descending order: Orbscan II, Pentacam HR, and MR-6000. Youn et al. [34] also reported that the Scheimpflug camera AL-scan (Nidek, Gamagori, Japan) tended to measure thicker than that with the Pentacam, with a difference of 5 to 6 μm on average, which may not be clinically meaningful. Likewise, in our study, CCT was significantly different between the MR-6000 and other devices. On comparing MR-6000 with Pentacam HR, the mean difference was −7.53 μm. Despite a significant p-value, the ICC showed an almost perfect agreement (ICC = 0.934). Considering both statistical results, clinical agreement existed between MR-6000 and Pentacam HR. MR-6000, Pentacam HR, and Orbscan II measured corneal thickness using light reflected from the posterior and anterior corneal surfaces. However, because this mechanism is not considered to be the most precise for measuring corneal thickness, and thus far, the standard for measuring corneal thickness is US pachymetry [35]; a limitation in this study is that the comparison was performed excluding measurement by US pachymetry.
WTW distance is an important factor in determining the size of the posterior chamber phakic intraocular lens and is used to refer to the horizontal distance of the ciliary sulcus [36,37]. Previous studies comparing the WTW distance measured by various devices found that the WTW distance measured by Orbscan II was smaller than that measured by IOLMaster [38,39]. Oh et al. [40] reported that the WTW distance measured by Orbscan II was not correlated with the WTW distance measured by 35-mHz ultrasound biomicroscopy (UBM). In this study, the WTW distance measured by MR-6000 showed a significant difference with that measured by Orbscan II, but not IOLMaster 700. In the ICC analysis, MR-6000 and IOLMaster 700 also showed almost perfect agreement (ICC = 0.812, p < 0.001). The WTW distance measured by MR-6000 is interchangeable with IOLMaster 700, but not with Orbscan II. The reason that the WTW value varies depending on the device may be because image processing varies with the device used [41]. The WTW distance was calculated as the value between the limbal area lying in the white sclera and the darker iris image. When using this principle, the interference of contours by eyelashes, eyelids, nose, and illuminance during the examination also influences the measurement. Considering that the actual relationship between the horizontal distance of the ciliary sulcus and WTW distance is controversial [42,43], and previous studies have reported inconsistent measurements of the WTW distance not by UBM but by topography or caliper, follow-up studies are needed to investigate the correlation between the WTW distance measured by other topography devices or UBM and the actual horizontal ciliary sulcus.
In the comparison of IOP measurements between MR-6000 and FT-1000, neither sample followed a normal distribution. Nonparametric Wilcoxon signed-rank test was performed, and a statistical difference was not observed between the IOP measured by MR-6000 and FT-1000. However, the distribution itself did not satisfy normality, and there was no ICC between them. Both devices use the concept of NCT based on the Imbert-Fick law. A pneumatic applanation tonometer measures the IOP without touching the eye. NCT decreases infection risk and is free from the mechanical influence of the cornea compared with Goldmann applanation tonometry (GAT) but is more susceptible to the effects of CCT than those of GAT [44,45]. This study did not prove any correlation between MR-6000 and the existing NCT, which may be because small populations are out of normal distribution, and because it is compared with NCT not with GAT, which is the gold standard for clinical IOP measurement. Since MR-6000 can obtain corrected IOP reflecting CCT measured by pachymetry, further studies comparing IOP by MR-6000 with GAT are needed.
To our knowledge, this is the first study to compare a new multifunctional anterior biometric unit with six existing devices. In the analysis of keratometry, CCT, WTW distance, and IOP, only keratometry using MR-6000 was interchangeable with the four other devices: KR-1, Pentacam HR, IOLMaster 700, and Orbscan II. CCT measured by MR-6000 was clinically interchangeable with Pentacam HR to some degree, but not with Orbscan II. The WTW distance measured by MR-6000 could be replaced with the WTW distance measured by IOLMaster 700, but not with that measured by Orbscan II. The IOP is not interchangeable with MR-6000 through the existing NCT device (FT-1000).
This study had a few limitations owing to its retrospective design. First, the number of study participants was relatively small, and only adult patients who were 38 to 83 years of age and had cataracts were included. The study did not reflect the severity of cataracts, and adaptation of the results to adults younger than 38 years and children is not convincing. However, with normality tests and based on the assumption of normality that if the sample is based on 30 or more observations, the sample distribution of the mean can be safely assumed to be normal [46], correlation and agreement analyses are possible, and these are statistically reliable. Second, due to the retrospective nature of the study, we could not include gold standard measurements, such as WTW distance measured by UBM and IOP measured by GAT. Third, we attempted to determine the correlation and agreement of MR-6000 with other devices; however, estimating the reproducibility of MR-6000 was not included in this study. In addition, although we recommended patients to blink before each measurement and set the identical condition before measurement, we could not exclude the factors such as dryness or erosion of cornea that may occur during the series of measurements and affect results.
The results of this study showed that even the newly developed multifunctional unit could not replace different devices, including topography, tonometry, and pachymetry, but showed great consistency in keratometry. Although it is not suitable to substitute MR-6000 for other devices in cataract preoperative examinations, MR-6000 has strengths in measuring multiple parameters at once. Considering that MR-6000 also equips dry eye evaluation, which was excluded in this study, MR-6000 may be utilized in ocular surface diseases during initial clinic visits for rapid screening with offering information of major ocular segment biometry. The strength of the MR-6000, which enables to get various accurate details in once by single device, will contribute to saving time and space with high cost-effectiveness. Further studies on other categories, such as patients with dry eye or patients planning refractive surgery, and the usefulness of MR-6000 as the first multifunctional unit of anterior ocular biometry can be reevaluated. Based on this retrospective cross-sectional study, futures studies also need to be mapped out prospectively to provide the information about cost-effectiveness and efficacy.
Acknowledgements
None.
Notes
Conflicts of Interest: None.
Funding: This work was supported by a faculty research grant of Yonsei University College of Medicine (No. 6-2020-0222), the Basic Science Research Program (No. NRF-2021R1I1A1A01047951) of the National Research Foundation (NRF), and by the Korean Fund for Regenerative Medicine (KFRM) grant (No. KFRM 22C0615L1) funded by the Korea government (the Ministry of Science and ICT, the Ministry of Health and Welfare). The funding organization had no role in the design or conduct of this study.