Novel Findings of Polypoidal Choroidal Vasculopathy via Optical Coherence Tomography Angiography
Article information
Abstract
Purpose
To introduce novel findings of polypoidal choroidal vasculopathy (PCV) via optical coherence tomography angiography (OCTA)
Methods
This study is a retrospective chart review of 16 patients (16 eyes) with PCV. OCTA (Avanti RTVue XR) findings were evaluated and selected for analysis after agreement by two retina specialists .
Results
Twenty one polyps in 16 eyes (16 patients) with PCV were included in this study. The mean patient age was 67 years (13 men and three women). The shape of polypoidal lesions on OCTA at initial were halo (five polyps), rosette (seven polyps), and vascular network (nine polyps). Eight months after anti-vascular endothelial growth factor treatment, in a total of four eyes, seven polyps could be followed up completely, the two halo type polypoidal lesions changed to rosette and vascular network type. The lesions of three rosette and two vascular network type lesions did not change in shape. In addition, the size of the polypoidal lesions (one among two halo types, two among three rosette types, and two among two vascular network types) decreased, but one halo type did not change and one rosette type increased in size on OCTA.
Conclusions
En-face OCTA enabled us to categorize novel types of PCV with polypoidal lesions.
Polypoidal choroidal vasculopathy (PCV) is a disease characterized by a branching vascular network in the choroid with terminal, polyp-like aneurysmal dilations. It was named by Yannuzzi et al. in 1990 [1]. Indocyanine green angiography (ICGA) is essential in the diagnosis of PCV. Typically, branching blood vessels of varying sizes form a network, and the extended polyps are shown at the end under retinal pigment epithelium [234]. PCV not only causes subretinal hemorrhage and retinal pigment epithelial detachment in patients aged 50 years or older like age-related macular degeneration, but also induces bleeding and exudative changes due to the active polyps without a precedent change in Bruch's membrane or soft drusen. It may cause permanent visual deterioration due to changes of retinal pigment epithelium, bleeding, and disciform scars through chronic progression. For this reason, treatment based on accurate diagnosis is necessary [356].
Although ICGA is a basic diagnostic method for PCV, there are many inconveniences and side effects associated with the examination which involves intravascular injection of the contrast agent. Optical coherence tomography (OCT) is also very useful because, polypoidal elevation of the retinal pigment epithelium, a distinct characteristic of PCV, can be observed. In addition, changes in retinal pigment epithelial detachment and subretinal f luid after treatment can be identified [789]. However, the disadvantage of OCT is difficulty in observing pathological changes in the blood vessels that are characteristics of PCV. As a result, it is not used as a basic examination for identifying PCV [10].
Recently, optical coherence tomography angiography (OCTA) has been introduced and used for the diagnosis of PCV, and for follow-up observation of progress after treatment. OCTA measures the movement of red blood cells in retinal and choroidal vessels over time through a non-invasive method and presents it as a B-scan of OCT. For this reason, it is possible to efficiently diagnose the change in vascular structure of each retinal layer, which is difficult to analyze with ICGA [11]. Most reports showed that the vascular network structure could be observed more clearly in OCTA, whereas better images of polyps were produced by ICGA [11121314151617]. In some reports, the types of polyps on ICGA were classified as solitary, clustered, and combined [18]. Furthermore, their prognoses were analyzed, solitary aneurysmal dilated lesions were stable with a favorable clinical course, but the cluster of grape-like lesions were active with leakage or hemorrhage that caused visual loss [6]. The shapes of the polyps found on OCTA were described as a bright round lesion or a bright outline with dark lumen [61112131415161718]. However, no studies have classified the shape of the polyp and analyzed changes after treatment on OCTA.
This study aimed to propose a new classification by determining the shape of the polyp found on OCTA, and to compare the change after treatment with that of ICGA according to the classification.
Materials and Methods
This study retrospectively analyzed the medical charts of 16 patients (16 eyes), that were diagnosed with PCV at the Retina Center, Nune Eye Hospital. The enrolled study eyes had one or more nodular extensions at the end of a branching vascular network (BVN) on ICGA, whose image quality of OCTA was clear enough for the study. About 8 months after anti-vascular endothelial growth factor (anti-VEGF) treatment, in a total of four eyes, seven polyps could be followed up completely. All enrolled patients underwent both ICGA and OCTA to allow for parallel comparison.
Basic ophthalmological examination, fundus photography, fluorescein angiography (FAG), ICGA, and OCTA were performed. FAG and ICGA were conducted with Heidelberg retinal angiography (HRA-2, Heidelberg Engineering, Heidelberg, Germany) using a confocal scanning laser ophthalmoscope. OCTA was conducted using the Avanti RTVue XR (Optovue Inc., Fremont, CA, USA) that used the split-spectrum amplitude-decorrelation angiography algorithm. PCV size was measured by the built-in programs of the HRA-2 and OCTA.
Polypoidal lesions on ICGA were classified into cluster and aneurysm according to morphology, and the change in lesion size after the treatment was measured along with the BVN. Moreover, PCV lesions identified on ICGA were analyzed on OCTA while changes related to their shapes, sizes, and treatments were measured. The pattern and change of the polypoidal lesions and BVN in both images were reviewed by two experienced doctors (SS and OWK). The study was conducted in adherence to the tenets of the Declaration of Helsinki and was approved by the institutional review board of Nune Eye Hospital (N-1802-001-999). Informed consent was waived by the board.
Results
The follow-up period of this study was 8.3 months (8 to 9 months), and in a total of four eyes, seven polyps could be followed up completely. During this period, patients underwent an average of 4.8 rounds of anti-VEGF therapy with aflibercept (Eylea; Bayer, Berlin, Germany). The age range of the patients was 57 to 83 years (mean age of 67 years old), and 13 men and three women were included.
Characteristics of polypoidal choroidal vasculopathy-PCV on ICGA and OCTA
There were 20 polyps found on ICGA and 21 polyps found on OCTA (Table 1). The reason for the difference was that one aneurysm-type polyp from case 4 on ICGA was observed as separated polyps on OCTA. In addition, two types of polyps were found on ICGA including cluster (four out of 20) and aneurysm types (16 out of 20). However, these polyps were observed in various patterns found on OCTA, including halo type (five out of 21) with a high-flow density surrounding the inner regular dark circular cavity and the periphery, vascular network type (nine out of 21) similar to BVN, and rosette type (seven out of 21) with a high-flow density surrounding the inner irregular dark cavity and the periphery (Fig. 1A–1L, 2A–2L, 3A–3L, 4A–4L). In terms of lesion size at initial examination, the mean size of polyps was 0.1 mm2 on ICGA and 0.22 mm2 on OCTA, thereby showing a significant difference between the two examinations (p = 0.014). Meanwhile, the mean size of BVN was 2.66 mm2 on ICGA and 2.15 mm2 on OCTA, and there was no significant difference (p = 0.372).
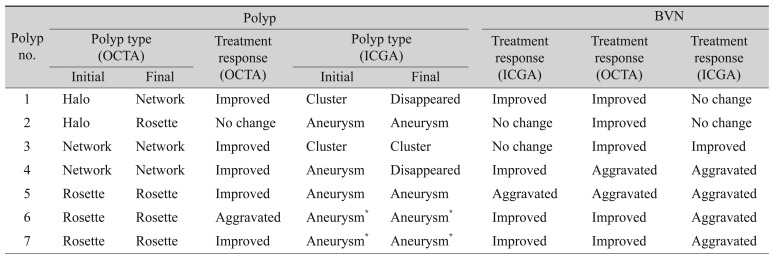
Case 1 indocyanine green angiography (ICGA), optical coherence tomography angiography (OCTA), En Face, and spectral-domain optical coherence tomography (SD-OCT) findings. (A) Two hot spots (cluster and aneurysm, white arrow and arrowhead) of polypoidal lesions and branching vascular network (BVN, black arrow) were detected on ICGA. (B) Two round and high-flow outlined with dark halo polypoidal lesions (arrow and arrowhead, yellow colored area) were detected on OCTA. (C) These polpypoidal lesions were also detected on En-Face image (cluster and aneurysm, white arrow and arrowhead). (D) BVN (arrow) was identified on OCTA. (E) Cluster type of polypoidal lesion was disappeared after treatment, but aneurysm type was still remained on ICGA (cluster and aneurysm, white arrow and arrowhead; BVN, black arrow). (F) Large-sized halo type of polyp (arrow) was changed to vascular network type, and small-sized halo type polyp (arrowhead) was changed into rosette type on OCTA. (G) En-Face finding of polyps after treatment (cluster and aneurysm, white arrow and arrowhead). (H) Decreased the area of BVN (arrow) on OCTA. (I,J) SD-OCT finding of polyps (arrow) at initial and after treatment. (K,L) 3D finding of polyp at initial and after treatment.

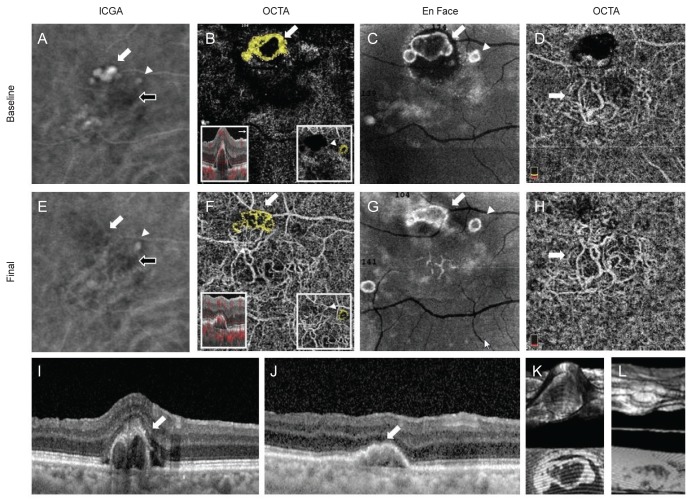
Case 2 indocyanine green angiography (ICGA), optical coherence tomography angiography (OCTA), En Face, and spectral-domain optical coherence tomography (SD-OCT) findings. (A) Cluster type of polypoidal lesion (white arrow) and branching vascular network (BVN, black arrow) were detected on ICGA. (B) High-flow vascular network polypoidal lesions (white arrow and yellow colored area) was detected on OCTA. (C) This polpypoidal lesion (white arrow) was also detected on En-Face image. (D) BVN (arrow) was identified on OCTA. (E) Decreased size of cluster type polypoidal lesion (white arrow) and BVN (black arrow) after treatment on ICGA. (F) Vascular network type of polyp (white arrow) was maintained as the original type on OCTA. (G) En-Face finding of polyp (white arrow) after treatment. (H) Decreased the area of BVN (arrow) on OCTA. (I,J) SD-OCT finding of polyps (arrow) at initial and after treatment. (K,L) 3D finding of polyp at initial and after treatment.
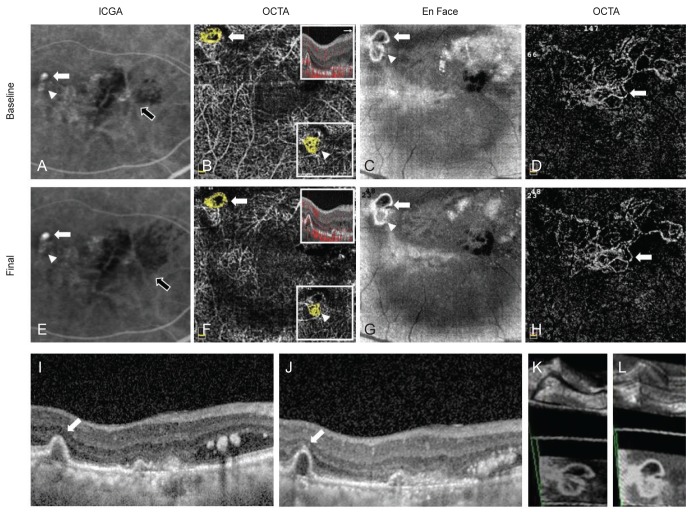
Case 3 indocyanine green angiography (ICGA), optical coherence tomography angiography (OCTA), En Face, and spectral-domain optical coherence tomography (SD-OCT) findings. (A) Two hot spots (aneurysms, white arrow and arrowhead) of polypoidal lesions and branching vascular network (BVN, black arrow) were detected on ICGA. (B) High-flow outlined with irregular dark halo, the rosette type, polypoidal lesions (arrow) and high-flow vascular network type polypoidal lesion (arrowhead, in the small box) were detected on OCTA (yellow colored area). (C) These polpypoidal lesions (white arrow and arrow head) were also detected on En-Face image. (D) BVN (arrow) was identified on OCTA. (E) The brighter and large-sized aneurysm type of polypoidal lesion (white arrow) was increased the size, but the relatively faint aneurysm type (arrowhead) was almost disappeared after treatment on ICGA (BVN, black arrow). (F) The rosette type (arrow) and the vascular network type (arrowhead, in the small box) were unchanged as the original types after treatment on OCTA. (G) En-Face finding of polyps (white arrow and arrow head) after treatment. (H) Increased the area of BVN (arrow) on OCTA after treatment. (I,J) SD-OCT finding of polyps (arrow) at initial and after treatment. (K,L) 3D finding of polyps at initial and after treatment.
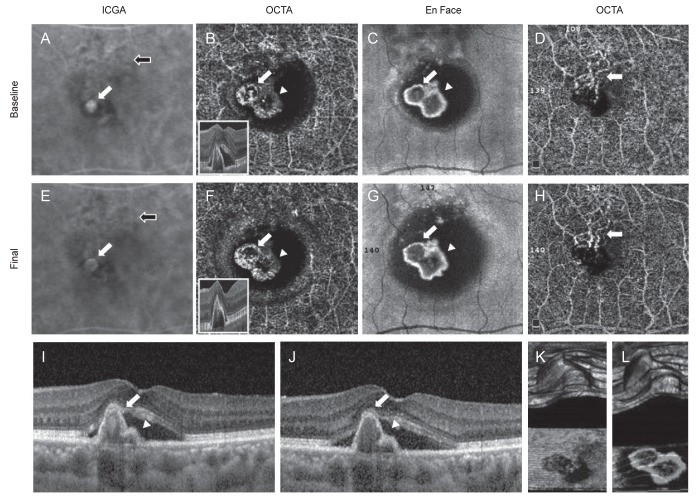
Case 4 indocyanine green angiography (ICGA), optical coherence tomography angiography (OCTA), En Face, and spectral-domain optical coherence tomography (SD-OCT) findings. (A) One hot spots (aneurysm, white arrow) of polypoidal lesion and branching vascular network (BVN, black arrow) were detected on ICGA. (B) Two round and high-flow outlined with irregular dark halo polypoidal lesions (arrow and arrowhead) were detected on OCTA. (C) These polpypoidal lesions (white arrow and arrowhead) were also detected on En-Face image. (D) BVN (arrow) was identified on OCTA. (E) The fluorescence of aneurysm type of polypoidal lesion (white arrow) was decreased size after treatment (BVN, black arrow). (F) Two rosette type polyps (arrow and arrowhead) were remained as the original types after treatment on OCTA. (G) En-Face finding of polyps (white arrow and arrowhead) after treatment. (H) Decreased the area of BVN (arrow) on OCTA after treatment. (I,J) SD-OCT finding of polyps (white arrow and arrowhead) at initial and after treatment. (K,L) 3D finding of polyps at initial and after treatment.
Longitudinal study of PCV on ICGA and OCTA
Eight months after anti-VEGF treatment, in a total of four eyes, were followed up. Based on longitudinal changes in the shape of the polypoidal lesion after treatment, a total of six polyps observed on ICGA either maintained their original type at final follow-up observation (four polyps) or the polyp disappeared (two polyps), while five of seven polyps found on OCTA maintained their original type, and two of seven polyps showed a change in type after treatment (Table 2). These changes in shape appeared in the two halo types, and they were changed to vascular network type and rosette type, respectively. In terms of treatment response according to type found on OCTA, one of two halo types changed into a vascular type with decreased size, thereby showing improvement in its pattern while another polyp was changed into a rosette type without any change in size. All vascular network types maintained their original types at the final follow-up observation, and the sizes of the polyps were reduced. All rosette types maintained their original type. However, the size of the lesion was reduced in two of three cases and was increased in the rest of the lesions that showed a worsening pattern. In terms of BVN connected with each polyp, the BVN size was reduced after the treatment in all cases of PCV with halo-type polyps. In half of the cases of PCV with vascular network type polyps, the size of BVN was reduced. However, the size of BVN increased in the remaining half. For the rosette type, the size of BVN was reduced in two of three cases, but the size of BVN increased in the remaining one of three cases. During the study period, initial visual acuity was unchanged except for case 1, which showed only a slight improve in best-corrected visual acuity from 20 / 25 to 20 / 20.
Discussion
PCV is a disease characterized by branching vascular network, terminal polyp-like aneurysmal dilations, and exudative changes. It is also known for having a relatively good natural prognosis, unlike choroidal neovascularization accompanied by age-related macular degeneration [16]. However, during long-term follow-up, recurrence is common and chronic progression occurs because BVN develops a new polyp at the ends as it grows [35].
The BVN of PCV is well demonstrated on ICGA at an early phase, hyperfluorescence of the polyp type is remarkably observed during the middle phase, and the number of polyps varies from 2 to 8 [21920]. According to previous studies, PCV polyps found on OCTA could be confirmed by ICGA in approximately 50% to 100% of cases, and BVN could be confirmed in approximately 70% to 100%. Polyps are observed to be significantly smaller in size on OCTA than on ICGA. In the case of BVN, an extremely bright vascular structure can be distinguished from the surrounding normal blood vessels, and a clear boundary can be identified, as compared to ICGA [1112131415161721]. It has also been reported that polyps were observed as various flow signals on OCTA, such as a bright round lesion or a bright outline with dark lumen. However, in this study, polyps were observed in three patterns on OCTA including the halo type with a high-flow density surrounding the inner regular dark circular cavity, the vascular network type similar to BVN, and the rosette type with a high-flow density surrounding the inner irregular dark cavity (Fig. 1, 2, 3, 4) [61112131415161718]. However, it is difficult to determine which of these various polyps are active polyps that can cause symptoms and inactive polyps that often change into active polyps within a short period of time [68222324252627282930]. In the case of BVN, it is known that there is no significant change in the maximum diameter before and after the treatment in all cases, even though there is significant improvement in corrected visual acuity, complete remission of subretinal f luid, significant decrease in the mean macular and choroidal thickness, and loss of polyp after intravitreal anti-VEGF injection. For this reason, it is challenging to accurately identify current lesion activity status [22232425].
Based on previous reports on PCV treatment, photodynamic therapy shows a n over 70% nodal closure rate as well as improvement in visual acuity and FAG findings. Nevertheless, recurrence is common after one year and there is risk of macular ischemia, subretinal hemorrhage, and pigment epithelial atrophy [2430]. Intravitreal anti-VEGF injection is increasingly common and is known as an effective treatment for reducing exudation and maintaining visual acuity. However, the effect is limited, since it has a nodal closure rate of 20% to 40% [26272829]. The recently developed aflibercept has a nodal closure rate of 50% to 75% [222330]. Based on various reports, polyps, SRF, and PED improved after anti-VEGF therapy, but BVN was maintained without any change [22232425]. The subjects of this study have never received photodynamic therapy, aflibercept was injected more than once in all cases except for case 4, and only bevacizumab was injected into case 4. As a result, the sizes of 6 of the 7 polyps on OCTA were reduced at final follow-up observation (p = 0.124), and for BVN, improvement was shown in 3 of the 4 cases, although it was not significant (p = 0.519) (Table 2).
Tanaka et al. [17] classified the PCV polyps into two types according to location, and reported that the shape of the polyp could be observed differently on OCTA. For this reason, either hemorrhagic pigment epithelial detachment (PED) or subretinal hemorrhage was suggested. This was only an analysis of type according to the polyp and anatomical situations of the surroundings at a specific point in time rather than an analysis of polyp activity. On the other hand, this study was conducted to clarify novel findings of the shape of polyps and to analyze the correlation of activity with the shape of the polyps.
In this study, polyps, observed as a single or clustered circular hyperfluorescence on ICGA, were found to have various shapes (e.g., halo, vascular network, and rosette) on OCTA. Based on the progress of the three types of polyps after treatment, the size of one of the two halo types was reduced at final observation while the size of the other polyp did not show any change. Two of the three rosette types had decreased size, but the size of the remaining rosette type increased (Table 2). However, sizes of the two vascular network types both decreased at final follow-up observation. The differences in the morphology of the polyps were found on OCTA and there were variable responses to treatment. Although the number of polyps that were analyzed in this study was limited to observing the tendency of changes, there was the possibility of various treatment responses by different types of polyps on OCTA. Long-term follow-up in more subjects is needed to evaluate treatment response according to the type of polyp and/or to analyze the accompanying changes in BVN using OCTA.
This study has several limitations. First, it is a retrospective study. Second, the sample size was small due to simultaneously performing ICG, OCT, and OCTA. Only patients who were observed for a sufficient period of time were selected. Finally, the shapes of a polypoidal lesion could be miscalculated, as they have a three-dimensional structure. For this reason, we tried to analyze every section of OCTA and examined the three-dimensional virtual image using the built-in program (K and L of Fig. 1, 2, 3, 4). Nonetheless, this study was meaningful in that the halo, rosette, and vascular network types of polyps were newly proposed in PCV patients via OCTA, a result which could not be observed on existing ICGA. These morphological differences may be correlated with treatment response.
Notes
This paper has been presented in 40th Annual Macula Society Meeting in Singapore, June 7, 2017, and APVRS in Malaysia, December 8, 2017.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.