 |
 |
| Korean J Ophthalmol > Volume 33(6); 2019 > Article |
Abstract
Purpose
To describe the clinical features of Korean patients with contact lens-induced limbal stem cell deficiency (CL-LSCD).
Methods
Medical records of 22 patients who were diagnosed with CL-LSCD between 2014 and 2019 were reviewed retrospectively. Outcome measures included demographics, clinical presentation, treatment, clinical course, and pattern of contact lens (CL) wear.
Results
Forty-two eyes of 22 patients were found to have typical changes associated with CL-LSCD. Twenty (91%) patients were women and mean age was 36 ┬▒ 12 years. All patients had myopia with mean spherical equivalent of ŌłÆ7.52 ┬▒ 3.2 diopter. Twenty (91%) patients had bilateral disease and the location of limbal involvement was diffuse in 20 eyes (47.6%) and partial in 22 eyes (52.4%, superior in 20 eyes and inferior in 2 eyes). Fourteen (63.6%) patients complained of decreased visual acuity. Average period of CL wear was 14 ┬▒ 9 years. Four patients used cosmetic colored CLs and four patients had a history of overnight CL wear. All 12 patients who completed follow-up (28 ┬▒ 42 weeks) showed improvement in visual acuity and ocular surface condition after cessation of CL wear and medical treatment. Of them, five (42%) patients showed full recovery while seven (58%) showed partial recovery.
Conclusions
If a patient with a history of CL wear for an extended period of time presents with decreased visual acuity, practitioners should perform detailed examinations with suspicion of CL-LSCD, including fluorescein staining. CL-LSCD is usually reversible and close follow-up with conservative treatment is recommended as the initial treatment option.
Limbal epithelial stem cells (LSCs) maintain the corneal surface by providing precursors that differentiate and replace the corneal epithelium [1]. In addition, these cells act as a barrier to prevent overgrowth of conjunctival tissue toward the cornea [2]. Limbal stem cell deficiency (LSCD) is caused by destruction and/or dysfunction of precursors of the corneal epithelium in the basal limbal area, resulting in conjunctival epithelial growth onto the corneal surface [3].
There are numerous etiologies of LSCD, including congenital anomalies (aniridia and ectodermal dysplasia) and acquired diseases (Stevens-Johnson syndrome, cicatricial pemphigoid, and chemical, thermal, or surgical traumatic injury). Contact lens (CL) wear is a relatively less well-known cause of LSCD. As CL-induced LSCD (CL-LSCD) is often asymptomatic, it can be easily missed [4]. A retrospective study reported that 2.4% of soft CL wearers had focal LSCD findings, with only 28.6% of wearers reporting subjective disease-related symptoms [5].
Recently, Chan and Holland [6] reported a case series study of severe LSCD from CL wear in which 78% of eyes underwent LSC transplantation, whereas Kim et al. [7] reported a case series of patients with medically reversible LSCD, 72% of whom had CL-LSCD. There are few studies of CL-LSCD [5,8,9] and no reports of CL-LSCD in Korean subjects to the best of our knowledge. The purpose of this study was to describe the clinical features of Korean patients with CL-LSCD and the pattern of CL wear in these patients.
Medical records of patients diagnosed with CL-induced LSCD at a tertiary referral center in South Korea between January 2014 and June 2019 were reviewed retrospectively. The study was conducted according to the tenets of the Declaration of Helsinki and the institutional review board of Seoul National University Bundang Hospital granted approval for collecting the clinical data used in this research with waiver of informed consent (B-1908-556-101).
Only patients who had history of CL wear and no other well-known causes of LSCD were included. Because impression cytology was not available due to the retrospective nature of the study, diagnosis of LSCD was made based on typical clinical findings suggestive of CL-LSCD: loss of well-defined palisades of Vogt, whorl-like epitheliopathy with late fluorescein staining, and superficial corneal neovascularization [7]. Diagnosis was made by two cornea specialists (JYH and HSJ) at presentation and confirmed by a cornea specialist (HSJ) on review of recorded anterior segment photographs. Patients who had coexisting potential causes for LSCD, such as ocular injury (chemical or thermal), prior ocular surgery, systemic disease that can affect the ocular surface (Stevens-Johnson syndrome, cicatricial pemphigoid), or long term use of topical glaucoma medications were excluded.
Demographics and clinical features such as chief complaint, initial best-corrected visual acuity, refractive error as spherical equivalent, ocular examination findings including limbal disease location and laterality, treatment interventions, final best-corrected visual acuity, and clinical course of each patient were recorded. Location of limbal involvement was classified as diffuse or partial; diffuse refers to nearly total limbal involvement while partial refers to only isolated superior or inferior limbus involvement.
Additionally, CL wear history was investigated through medical records or telephone interviews with patients who were willingly to undergo an interview. CL wear history included duration of CL wear (years), hours of CL wear per day, CL replacement cycle, wearing of cosmetic colored CLs, and overnight use.
Data were analyzed using IBM SPSS Statistics ver. 22.0 (IBM C orp., Armonk, NY, USA) a nd a re presented a s means ┬▒ standard deviations.
Forty-two eyes of 22 patients were found to have typical clinical changes associated with CL-LSCD. Clinical features of the patients are summarized in Table 1. Mean age of patients was 36 ┬▒ 12 years (range, 18 to 65 years) and 20 of 22 (91%) patients were female. All patients had myopia with a mean spherical equivalent of ŌłÆ7.52 ┬▒ 3.20 diopters (range, ŌłÆ0.50 to ŌłÆ16.25 diopters).
All eyes with CL-LSCD had irregular epitheliopathy extending from the limbus. Various epithelial staining patterns were observed, with the most common pattern a whorl-like and wavy pattern (Fig. 1A-1L). Of the 22 patients, 20 (91%) had bilateral manifestation and two (9%) had unilateral manifestation. Location of limbal involvement was estimated clinically and classified as diffuse or partial. Diffuse involvement was seen in 20 (47.6%) eyes and partial involvement in 22 (52.4%) eyes, with only two eyes showing inferior limbal involvement while the rest showed superior limbal involvement.
Patients complained of various symptoms including blurred vision, eye pain, and hyperemia. Fourteen (63.6%) patients had decreased visual acuity: all patients with diffuse involvement showed decreased visual acuity, while 5 of 12 (41%) patients with partial involvement had decreased visual acuity.
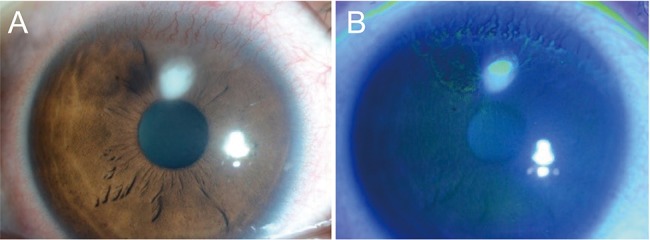
There was one case (patient 15) of ulcerative keratitis adjacent to the area of LSCD at initial presentation (Fig. 2A-2F). We prescribed topical 0.5% moxifloxacin six times per day and topical 1% prednisolone acetate four times per day and two days after initiation of treatment, the epithelial defect size and extent of infiltration had decreased. The patient was lost to follow-up and we could therefore not confirm full recovery.
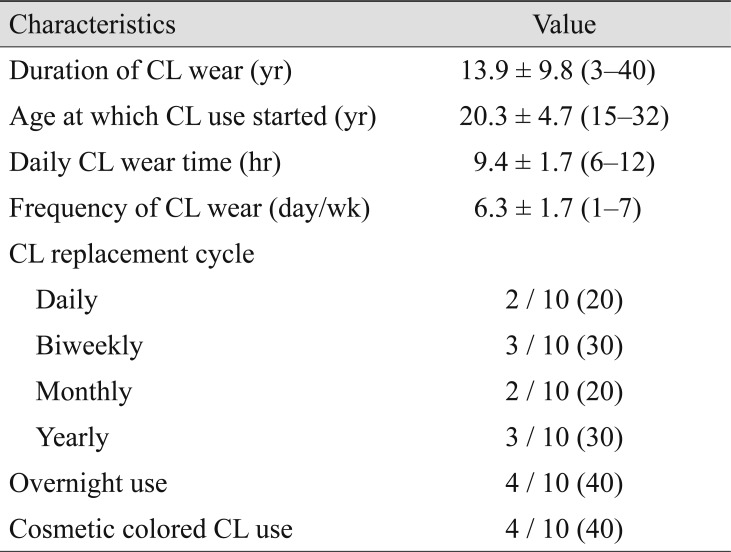
The CL wear histories of patients are summarized in Table 2. Duration of CL wear and the age when CLs were first worn were investigated through medical records in 18 patients. Ten of 22 (45%) patients agreed to a telephone interview and described their CL wear history more specifically. Patients wore CLs for 14.3 ┬▒ 12.1 years on average (range, 3 to 40 years), and the mean starting age was 20.3 ┬▒ 4.7 years (range, 15 to 32 years). On average, patients wore CLs for 6.3 ┬▒ 1.7 days per week and 9.4 ┬▒ 1.7 hours per day. Four of 10 (40%) patients had a history of overnight CL use and 4 of 10 (40%) described cosmetic colored CL usage. Various CL replacement cycle were recorded, varying from daily (2 / 10, 20%) or biweekly (3 / 10, 30%) to monthly (2 / 10, 20%) and yearly (3 / 10, 30%). Most patients did not remember the specific brand of their CLs or the name of the cleaning solution they used.
After the diagnosis of LSCD, all patients were advised to stop wearing CLs. Medical treatments such as artificial tears, autologus serum, and topical corticosteroids were prescribed to patients on an individual basis depending on the severity of the disease. Twelve of 22 (54%) patients were followed-up for observation (28 ┬▒ 42 weeks) while the remaining 10 patients were lost to follow-up after the first visit. At the final presentation, all 12 patients showed improvement in visual acuity and/or ocular surface condition. Five patients (42%) showed full recovery without any signs of CL-LSCD while seven patients (58%) showed improved epitheliopathy with only subtle subepithelial opacity and partial whorl-like epitheliopathy just adjacent to limbal area. Representative cases are presented in Fig. 3A, 3B.
In this study, we investigated 42 eyes of 22 patients who were diagnosed with CL-LSCD. To our knowledge, this is the first report of CL-LSCD in South Korea and the largest case series ever reported about CL-LSCD. Most patients were women (91%), had a long history of CL use (average duration of use was 14.3 years with 9.4 hours per day of wear), and had high myopia (mean spherical equivalent, ŌłÆ7.4 diopters). These results are similar to a previous study that reported these parameters as risk factors for CL-LSCD [5].
Decreased visual acuity was reported by 14 (63.6%) patients with central cornea involvement. This might be the first symptom to motivate a patient with CL-LSCD to visit an ophthalmologist. Therefore, a patient who has used CLs for a long period of time who presents with decreased visual acuity should be suspected of having CL-LSCD. Findings on slit lamp examination varied widely from subtle epitheliopathy to a diffuse irregular surface. Whorl-like epitheliopathy and surface irregularity can be missed without careful examination after f luorescein staining combined with a cobalt blue filter. It is also important to examine the superior peripheral cornea covered by the upper eyelid, as this is the most frequently involved area. There was one case of ulcerative keratitis adjacent to the whorl-like irregular epithelium (Fig. 2). Delayed epithelial healing in patients with LSCD may be related to the presence of corneal ulcers.
We investigated the pattern of CL wear in some patients by telephone interview. Predictably, all patients had used CLs for a long time (Table 2). Almost half the patients (40%) had experience with overnight CL use. Most patients (70%) who stopped wearing CL due to CL-LSCD resumed CL wear after their complications resolved. Four of 10 patients (40%) wore cosmetic-colored lens for cosmetic purposes, not for medical purposes. This suggests that patients are unaware or not concerned about CL-related complications. A recent study reported that the age at which CLs are first worn is decreasing and the use of colored CLs for cosmetic purposes is increasing [10]. Therefore, CL wearers should be made aware that CL-related complications can affect ocular surface conditions and visual acuity considerably, and that regular eye exams are important.
When LSCD is suspected, it is important to stop CL wear immediately and to start medical treatment depending on the severity of the disease. Several studies have reported improvement in the disease without surgical treatment [7,9]. In our study, most patients who had central corneal epitheliopathy causing visual loss recovered in response to supportive care. No patients required surgical treatment such as amniotic membrane transplantation or LSC transplantation. In short, patients with CL-LSCD should be followed-up carefully with medical treatment. If there is no improvement, surgical treatment such as epithelial debridement, amniotic membrane transplantation, or limbal stem cell transplantation should be considered [9,11,12].
Understanding of the limbal niche, a special microenvironment associated with LSC function and deficiency, is increasing [13]. The function of LSCs and the limbal niche can be compromised by various insults to the ocular surface, including CL wear. Under certain conditions, these injuries can lead to dysfunction of LSCs a nd the limbal niche, but this dysfunction is reversible. If damage accumulates or is severe, permanent functional impairment can occur [13,14]. All patients in our series showed improvement of visual acuity and the ocular surface, but some of them recovered only partially; patients with diffuse involvement except for one patient and some patients with partial involvement showed remaining whorl-like epitheliopathy on the superior limbus and/or corneal subepithelial opacities. Early intervention in eyes with CL-LSCD may prevent progression to irreversible changes associated with LSC dysfunction.
There are several limitations to our study. First, diagnosis was based only on clinical findings on slit lamp examination, not impression cytology, which provides more definite confirmation of d iagnosis [5,15]. However, eyes with CL-LSCD showed typical findings of LSCD on slit lamp examination, making diagnosis straightforward. Second, 10 of 22 patients did not complete the follow-up, consistent with a previous study that reported that most CL users did not return for follow-up regularly as had been recommended [16]. Lack of awareness of disease severity could be related to follow-up loss or the patients' symptoms may have improved to such an extent after the first visit that they no longer felt the need to be examined and/or treated.
In conclusion, CL-LSCD can be diagnosed by typical findings on slit lamp examination with fluorescein staining, and is usually reversible. It is important to educate CL wearers that it is important to undergo regular ophthalmic examinations to prevent development of irreversible changes, as most early stages of CL-LSCD can be treated by cessation of CL wear and medical treatment. More research is needed to determine the prevalence of the disease and to establish a standard treatment regimen.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
REFERENCES
1. Thoft RA, Wiley LA, Sundarraj N. The multipotential cells of the limbus. Eye (Lond) 1989;3(Pt 2):109-113.


3. Dua HS, Azuara-Blanco A. Autologous limbal transplantation in patients with unilateral corneal stem cell deficiency. Br J Ophthalmol 2000;84:273-278.



4. Rossen J, Amram A, Milani B, et al. Contact lens-induced limbal stem cell deficiency. Ocul Surf 2016;14:419-434.



5. Martin R. Corneal conjunctivalisation in long-standing contact lens wearers. Clin Exp Optom 2007;90:26-30.


6. Chan CC, Holland EJ. Severe limbal stem cell deficiency from contact lens wear: patient clinical features. Am J Ophthalmol 2013;155:544-549.


7. Kim BY, Riaz KM, Bakhtiari P, et al. Medically reversible limbal stem cell disease: clinical features and management strategies. Ophthalmology 2014;121:2053-2058.



8. Bloomfield SE, Jakobiec FA, Theodore FH. Contact lens induced keratopathy: a severe complication extending the spectrum of keratoconjunctivitis in contact lens wearers. Ophthalmology 1984;91:290-294.


9. Jeng BH, Halfpenny CP, Meisler DM, Stock EL. Management of focal limbal stem cell deficiency associated with soft contact lens wear. Cornea 2011;30:18-23.


10. Kim JH, Song JS, Hyon JY, et al. A survey of contact lens-related complications in Korea: the Korean Contact Lens Study Society. J Korean Ophthalmol Soc 2014;55:20-31.

11. Tseng SC, Prabhasawat P, Barton K, et al. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol 1998;116:431-441.


12. Shen C, Chan CC, Holland EJ. Limbal stem cell transplantation for soft contact lens wear-related limbal stem cell deficiency. Am J Ophthalmol 2015;160:1142-1149.


13. Li W, Hayashida Y, Chen YT, Tseng SC. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res 2007;17:26-36.



14. Pajoohesh-Ganji A, Pal-Ghosh S, Tadvalkar G, Stepp MA. Corneal goblet cells and their niche: implications for corneal stem cell deficiency. Stem Cells 2012;30:2032-2043.



Fig.┬Ā1
Typical clinical appearances of contact lens-induced limbal stem cell deficiency on slit lamp microscopy without and with fluorescein staining. Typical whorl-like epitheliopathy with loss of normal corneal clarity and late fluorescein staining in a whorl-like pattern with adjacent punctate staining were seen in all patients with various surface irregularities. (A,B) Patient 2, (C,D) patient 5, and (E,F) patient 3 had diffuse limbal stem cell deficiency; (G,H) patient 12 and (I,J) patient 13 had partial superior limbal involvement; (K,L) patient 11 had partial inferior limbal involvement.
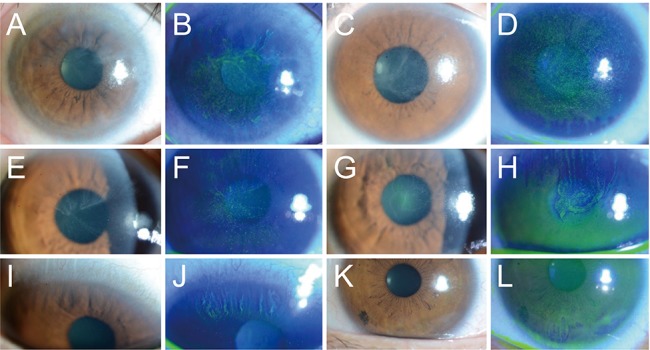
Fig.┬Ā2
Clinical courses of representative cases with contact lens (CL)-induced limbal stem cell deficiency (A-C) at first presentation and (D-F) after medical treatment. (A) A 22-year-old woman (patient 9) with a 6-year history of CL wear showed typical whorl-like epitheliopathy involving the pupil area. (D) Five months after cessation of CL wear and medical treatment, the previous lesion had disappeared. (B) A 40-year-old woman (patient 4) with a 20-year history of CL wear showed diffuse CL-induced limbal stem cell deficiency. (E) Six weeks after treatment, the center surface irregularity had recovered although there was remaining superior limbal deficiency and subepithelial opacity. (C) A 47-year-old woman (patient 3) with a 20-year history of CL wear showed superior and inferior whorl-like epitheliopathy. (F) Three months after treatment, the center epitheliopathy had improved and only peripheral limbal deficiencies remained.
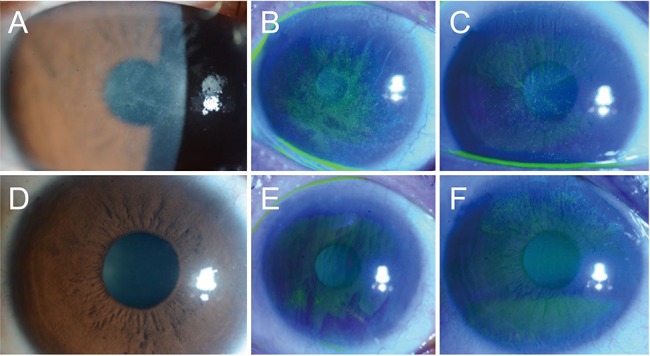
Fig.┬Ā3
A case of ulcerative keratitis in a patient with contact lens-induced limbal stem cell deficiency (patient 15). (A,B) Superior epithelial defect with infiltration adjacent to whorl-like irregular epithelium.
Table┬Ā1
Clinical features of 22 patients with contact lens-induced limbal stem cell deficiency
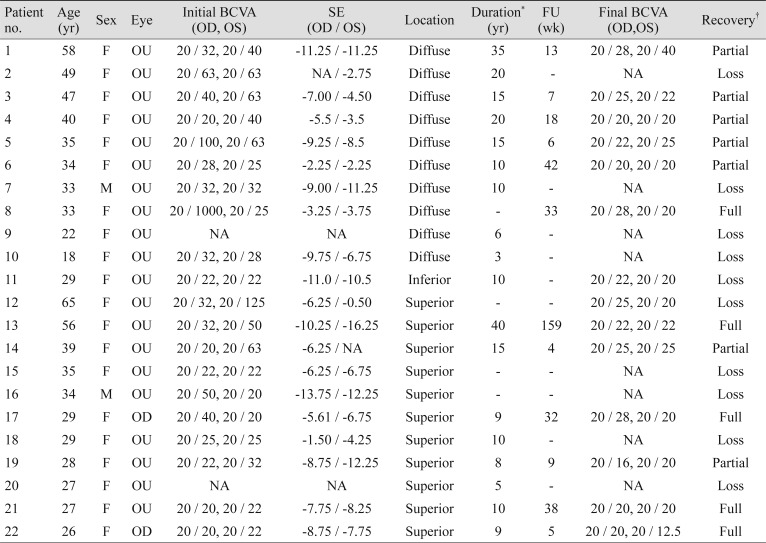
BCVA = best-corrected visual acuity; OD = right eye; OS = left eye; SE = spherical equivalent; FU = follow-up; NA = not applicable.
*Duration of contact lens wear (years); ŌĆĀFull = full recovery without any signs of limbal stem cell deficiency and improvement of visual acuity, Partial = partial recovery of ocular surface condition and/or visual acuity, Loss = patients lost to follow-up after first visit.
- TOOLS
-
METRICS

-
- 6 Crossref
- 0 Scopus
- 2,894 View
- 45 Download
- Related articles
-
Prevalence and Clinical Features of Sagging Eye Syndrome in Korean Patients2022 April;36(2)
Ophthalmologic Clinical Features of Facial Nerve Palsy Patients2019 February;33(1)



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



