Glaucoma drainage devices (GDDs) have been introduced to control intraocular pressure (IOP), especially in eyes with refractory glaucoma such as neovascular glaucoma and uveitic glaucoma, and in eyes that have failed glaucoma surgery. Theoretically, the amount of aqueous drainage through the GDD is determined based on its f luid dynamics profile. Thus, GDDs have to offer standardized and predictable control of IOP. However, despite the use of temporal ligation of the tube, staged operations, and valve-type GDDs, postoperative ocular hypotony due to excessive aqueous drainage still occurs [
1,
2,
3,
4,
5,
6,
7].
To overcome this limitation, we introduced two types of membrane-tube (MT) type glaucoma shunt devices (MT-device; MicroMT, using a 100 ┬Ąm internal diameter tube with a 7-0 nylon intraluminal stent and Finetube MT, using a 200 ┬Ąm internal diameter tube with a 5-0 nylon intraluminal stent) [
8,
9]. MT-devices consist of 0.1-mm-thick expanded polytetrafluoroethylene membranes and small diameter silicone tubes with intraluminal stents. These devices prevent postoperative ocular hypotony and provide stepwise reduction in IOP after surgery by retracting the stent [
8,
9]. During development of the MT-device, various
in vivo and
in vitro experiments were performed to determine the proper fluid dynamics profiles of tubes with safe (minimal risk of postoperative ocular hypotony) and effective (sufficient for IOP control) outflow characteristics [
10,
11,
12,
13,
14,
15]. For instance, when tubes of conventional GDDs (external diameter, 630 ┬Ąm; internal diameter, 300 ┬Ąm) were replaced with smaller tubes (external diameter, 300 ┬Ąm; internal diameter, 200 ┬Ąm), the replacements showed effective IOP control [
10,
11,
12]. In animal studies, the materials used in MT-devices had good biocompatibility [
13,
14], and in an experiment using a constant flow setting, the pressure levels of tubes with various diameters and materials were investigated [
15]. In the present study, we report the pressure and resistance of silicone tubes with intraluminal stents used in MT-devices at various steady-state flow rates.
Materials and Methods
Preparation of the tubes
Two types of silicone tubes, used in MicroMT [
8] and Finetube MT [
9] respectively, were investigated. These devices are still investigational and not yet commercially available. The tube used in MicroMT has a 200 ┬Ąm external diameter and 100 ┬Ąm internal diameter (Silicone no. 1; ARAM Micro Tubing, Japan) with a 7-0 nylon thread intraluminal stent. The tube used in Finetube MT has a 300-┬Ąm external diameter and a 200-┬Ąm internal diameter (Silicone no. 2, ARAM Micro Tubing), with a 5-0 nylon thread intraluminal stent (
Fig. 1). The MT-devices were designed to induce aqueous drainage from the anterior chamber to the post-limbal (MicroMT) and post-equatorial (Finetube MT) subconjunctival spaces, respectively. Therefore, the tube lengths used for MicroMT and Finetube MT were set at 5 and 10 mm, respectively. In this experiment, a total of 60 tubes (30 MicroMT and 30 Finetube MT) were used.
Devices for measuring pressure and resistance
Experiments were performed based on the experimental design described in the previous studies [
15,
16]. The instrument consisted of three components: a perfusion pump and syringe, a pressure transducer and detector, and a housing unit where the glaucoma implant was submerged in distilled water (
Fig. 1,
2). A perfusion pump with a stepper motor (Model 11; Harvard Apparatus, Holliston, MA, USA) was used. The perfusion pump was connected to a 1 mL glass syringe (Hamilton, Reno, NV, USA). A three-way stopcock was connected to the end of the glass syringe and calibration reservoir. A pressure transducer (PX260; Edwards Lifesciences, Irvine, CA, USA) and detector (8SP; ADInstruments, Colorado Springs, CO, USA) were connected to a personal computer, which monitored the pressure change in units of 0.01 mmHg using the Chart v5.1 (ADInstruments) software. We verified the pressure gauges whenever they were calibrated. A hole 1.5 cm from the base was made in the housing unit, and a 26-gauze needle sleeve was attached to the hole using silicone glue. The plastic cylinder, made from half of the plastic syringe used to fix the silicone tubes, was attached beneath the hole. The silicone tubes were submerged and connected to the pump and pressure transducer through the needle sleeve in the housing unit. The zero reference line was marked beyond the level of the hole (approximately 3 cm height from the base), and the pressure was measured as approximately 2.4 mmHg at that level. The height of this fluid was maintained by suction to define a zero reference line. This height was selected because the level was easy to control in our housing unit (6 cm height for the plastic box) and the glaucoma implant could be submerged completely at that level. Resistance was calculated using Poiseuille's equation at flow rates of 0, 2, 5, 10, and 25 ┬ĄL/min. Assuming ideal fluid flow in a noncollapsible tube, the equation can be simplified to resistance = (P
in - P
out) / rate of steady flow. P
in was measured using the pressure transducer and P
out was set constantly submerging the apparatus under a fluid column at a given height as described above.
Measurement of pressure and resistance of the tubes
In the experiments, the perfusion rate was increased in a stepwise manner from 0 to 2, to 10, and to 25 ┬ĄL/min. Each pressure at each flow rate was recorded (
Fig. 3). Since we assumed the episcleral venous pressure in the human eye to be 6 mmHg, we corrected the baseline pressure to 6 mmHg. Then, the pressure at each flow rate was corrected by adjusting the baseline pressure. For example, if the baseline pressure was 2.5 mmHg, we added 3.5 mmHg and set the baseline pressure as 6.0 mmHg. After that, we added 3.5 mmHg to the measured pressure at each flow rate level (2, 5, 10, and 25 ┬ĄL/min). The flow was maintained for up to 20 min at each step so that the resistance was calculated from steady-state flow and pressure conditions. Three-step experiments were performed in each tube: with a full-length intraluminal stent, after pulling the intraluminal stent by a half-length out of the tube, and after complete removal of the stent.
Statistical analysis
Friedman's test with post hoc analysis was used to compare the pressure and resistance among the tubes with a full-length intraluminal stent, with a half-length intraluminal stent, and without the intraluminal stent. In addition to the mean pressure and resistance level, the variance of pressure may provide information regarding the predictability of pressure; a lower variance may reflect a greater predictability. Therefore, to compare the pressure variance between the tubes with a full-length intraluminal stent, with a half-length intraluminal stent, and without an intraluminal stent, a Brown-Forsythe test was performed. All statistical analyses were performed using the PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA). A p-value less than 0.05 was considered statistically significant.
Results
In the tubes with MicroMT, the mean corrected pressures with a full-length intraluminal stent were 13.2 ┬▒ 3.0, 24.6 ┬▒ 8.1, 43.8 ┬▒ 16.5, and 98.1 ┬▒ 40.8 mmHg at flow rates of 2, 5, 10, and 25 ┬ĄL/min, respectively. With a half-length intraluminal stent, the mean corrected pressures were 10.5 ┬▒ 2.4, 16.7 ┬▒ 5.1, 26.4 ┬▒ 8.6, and 54.7 ┬▒ 21.2 mmHg at flow rates of 2, 5, 10, and 25 ┬ĄL/min, respectively. Without the intraluminal stent, the mean corrected pressures were 6.4 ┬▒ 0.2, 7.0 ┬▒ 0.2, 8.0 ┬▒ 0.4, and 10.9 ┬▒ 0.9 mmHg at flow rates of 2, 5, 10, and 25 ┬ĄL/min, respectively. The mean outf low resistance ranged from 3.6 ┬▒ 1.5 to 3.8 ┬▒ 1.7 mmHg/┬ĄL/min with a full-length intraluminal stent, from 2.0 ┬▒ 0.9 to 2.2 ┬▒ 1.1 mmHg/┬ĄL/min with a half-length intraluminal stent, and was 0.2 ┬▒ 0.0 mmHg/┬ĄL/min without the intraluminal stent (p < 0.001, with full-length stent > with half-length stent > without stent by
post hoc analysis) (
Table 1).
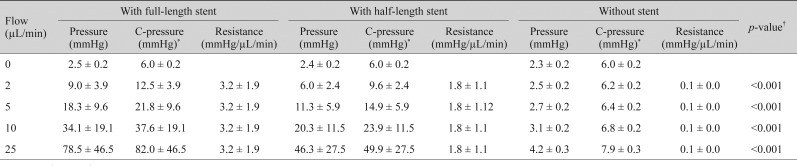
For Finetube MT, the mean corrected pressures with a full-length intraluminal stent were 12.5 ┬▒ 3.9, 21.8 ┬▒ 9.6, 37.6 ┬▒ 19.1, and 82.0 ┬▒ 46.5 mmHg at flow rates of 2, 5, 10, and 25 ┬ĄL/min, respectively. With a half-length intraluminal stent, the mean corrected pressures were 9.6 ┬▒ 2.4, 14.9 ┬▒ 5.9, 23.9 ┬▒ 11.5, and 49.9 ┬▒ 27.5 mmHg at flow rates of 2, 5, 10, and 25 ┬ĄL/min, respectively. Without the intraluminal stent, the mean corrected pressures were 6.2 ┬▒ 0.2, 6.4 ┬▒ 0.2, 6.8 ┬▒ 0.2, and 7.9 ┬▒ 0.3 mmHg at flow rates of 2, 5, 10, and 25 ┬ĄL/min, respectively. The mean outflow resistance ranged from 3.0 ┬▒ 1.9 to 3.2 ┬▒ 1.9 mmHg/┬ĄL/min with a full-length intraluminal stent, 1.8 ┬▒ 1.2 mmHg/┬ĄL/min with a half-length intraluminal stent, and 0.1 ┬▒ 0.0 mmHg/┬ĄL/min without an intraluminal stent (
p < 0.001, with full-length stent > with half-length stent > without stent by
post hoc analysis) (
Table 2).
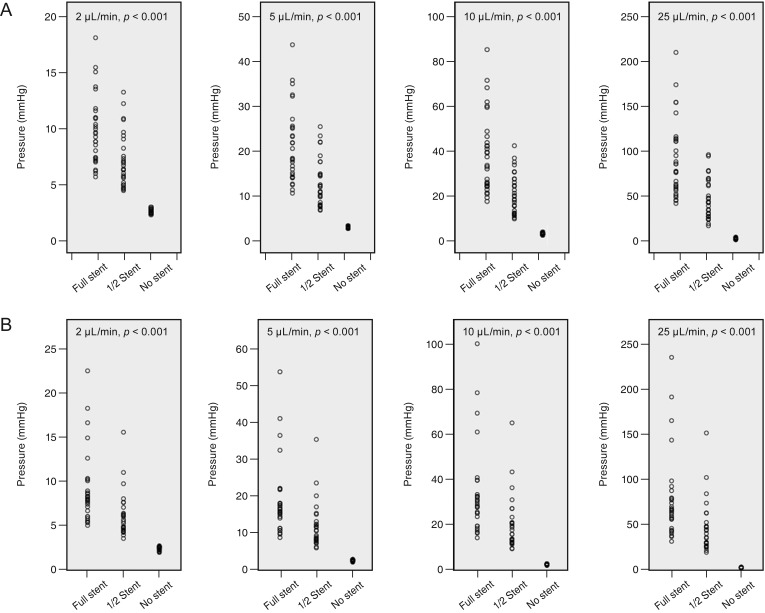
The pressure variances in the MicroMT and Finetube MT tubes are presented in
Fig. 4A and 4B. The pressure variances decreased based on the intraluminal stent retraction; the variance was highest with a full-length intraluminal stent and lowest without an intraluminal stent (
p < 0.001 at all flow rates).
Discussion
The present study demonstrated that silicone tubes with intraluminal stents used in MT-devices induced consistent and predictable pressures and resistances at various flow rates. In addition, when the intraluminal stent was partially and completely removed, the pressure and resistance gradually decreased. Therefore, GDDs using these tubes may contribute to safe and effective control of IOP with stepwise reduction.
Ocular hypotony is an important complication of GDD implantation, especially during the early postoperative period. To prevent this complication, various procedures have been introduced [
1,
2,
3,
4,
5,
6,
7]. One of these involves tube ligation with or without intraluminal or extraluminal stents during the surgery [
1,
2,
3,
4,
6,
7]. However, tube ligation may not produce a predictable pressure level; when the ligation is too tight or too loose, unexpected high or low pressure levels can occur, respectively. Other procedures, such as fenestrations or slit formations, are performed to prevent postoperative peaks in IOP after tube ligation [
4]. However, Rietveld and van der Veen [
17] showed that adjustable pressure regulation by focal tube constriction, similar to tube ligation, was disappointing because the maintenance of steady pressure levels at a given flow rate was difficult. They suggested that tube ligature to regulate pressure is trustworthy only with a highly sophisticated microarchitecture under well-controlled conditions.
Given that an intraluminal stent can induce occlusion in the entire area of the tube, it may provide more predictable pressure control compared to that of focal tube constriction. However, intraluminal stents have not been widely used. This may be because the silicone tubes of conventional GDDs, such as the Ahmed Glaucoma Valve (New World Medical, Rancho Cucamonga, CA, USA) and the Baerveldt Glaucoma Implant (Abbott Laboratories, Abbott Park, IL, USA), cannot provide the proper pressure to prevent ocular hypotony with the intraluminal stent because their internal diameters are too large. The inner diameters of the silicone tubes of the conventional GDDs are approximately 300 ┬Ąm. Therefore, a 3-0 nylon thread with a diameter between 200 and 250 ┬Ąm can be used as an intraluminal stent. However, results from a previous study show that the conventional silicone tube with a 3-0 polypropylene intraluminal stent do not successfully prevent ocular hypotony [
18]. Sheybani et al. [
19] also reported that a conventional tube with intraluminal 4-0 and 5-0 suture threads only provides pressures of 1.16 and 0.3 mmHg, respectively, at the flow rate of 2.5 ┬ĄL/min. Therefore, intraluminal stenting of conventional tubes by using 3-0, 4-0, and 5-0 suture materials may not prevent ocular hypotony. The 2-0 nylon thread with a diameter of 300 to 350 ┬Ąm may completely obstruct the lumen of the tube, but it is not easily inserted or may not be insertable into the lumen. For this reason, GDDs with an intraluminal stent have not been widely used for the prevention of postoperative ocular hypotony. Therefore, we hypothesized that a smaller tube with an intraluminal stent is a good alternative to conventional tubes.
The results from the present study demonstrated that for a physiologic flow rate of 2 ┬ĄL/min with an episcleral venous pressure of 6 mmHg, the mean pressures formed by the tubes of MicroMT were 13.2 and 10.5 mmHg with full-length and half-length intraluminal stents, respectively. With the tubes of Finetube MT, the mean pressures were 12.5 and 9.6 mmHg with full-length and half-length intraluminal stents, respectively. These pressure levels may be appropriate for the control of IOP with a minimal risk of ocular hypotony. When the flow rate increased, the pressure increased accordingly. However, the resistance remained consistent irrespective of the flow rate. In addition, when the intraluminal stents were partially and completely removed, the variance of pressure also decreased. This finding may be explained by the level of pressure; a higher level may tend to have a higher variance. These results suggest that the tubes of MT-devices may confer a consistent and predictable IOP level. To validate these
in vitro experimental results, we analyzed the clinical data of MT-devices and found that 1 year after the surgery, mean IOP decreased from a preoperative value of 23 to 15 mmHg after MicroMT implantation and from 33 to 17 mmHg after Finetube MT implantation without ocular hypotony [
8,
9].
An additional advantage to using an intraluminal stent is that it can allow stepwise IOP control through retraction of the stent. When the tube is focally constricted by ligation, only two-staged pressure control is available through postoperative removal of the ligation. However, after ligation removal, IOP can drop abruptly, causing ocular hypotony. In contrast, by using small silicone tubes with intraluminal stents, sudden decreases in IOP can be prevented because the stent can be retracted in a stepwise manner while monitoring the IOP; in the present study, stent retraction induced a stepwise and gradual decrease in the mean and variance of pressure. After the implantation of the MT-device, the intraluminal stent can be retracted if the pressure needs to be lowered. Our clinical results show that retracting the intraluminal stent half the length of the tube after the operation reduced the IOP by an additional 3 to 5 mmHg, and complete removal of the stent 4 weeks after the operation induced an additional 40% reduction in IOP without ocular hypotony [
8,
9].
In conclusion, the tubes of an MT-device provided pressure and resistance sufficient for IOP control with a minimal risk of ocular hypotony. Furthermore, it could provide stepwise reduction of IOP by retraction of the intraluminal stent. Therefore, these tubes may be a useful option for safe and effective control of IOP.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
REFERENCES
1. Barton K, Feuer WJ, Budenz DL, et al. Three-year treatment outcomes in the Ahmed Baerveldt comparison study.
Ophthalmology 2014;121:1547-1557.



2. Bailey AK, Sarkisian SR Jr. Complications of tube implants and their management.
Curr Opin Ophthalmol 2014;25:148-153.


3. Trible JR, Brown DB. Occlusive ligature and standardized fenestration of a Baerveldt tube with and without antimetabolites for early postoperative intraocular pressure control.
Ophthalmology 1998;105:2243-2250.


4. Sherwood MB, Smith MF. Prevention of early hypotony associated with Molteno implants by a new occluding stent technique.
Ophthalmology 1993;100:85-90.


5. Tong L, Frazao K, LaBree L, Varma R. Intraocular pressure control and complications with two-stage insertion of the Baerveldt implant.
Ophthalmology 2003;110:353-358.


6. Kee C. Prevention of early postoperative hypotony by partial ligation of silicone tube in Ahmed glaucoma valve implantation.
J Glaucoma 2001;10:466-469.


7. Lee JJ, Park KH, Kim DM, Kim TW. Clinical outcomes of Ahmed glaucoma valve implantation using tube ligation and removable external stents.
Korean J Ophthalmol 2009;23:86-92.



8. Ahn BH, Hwang YH, Han JC. Novel membrane-tube type glaucoma shunt device for glaucoma surgery.
Clin Exp Ophthalmol 2016;44:776-782.


9. Han JC, Hwang YH, Ahn BH. Membrane-tube-type glaucoma shunt device for refractory glaucoma surgery.
Graefes Arch Clin Exp Ophthalmol 2017;255:163-169.


10. Kim C, Kim Y, Choi S, et al. Clinical experience of e-PTFE membrane implant surgery for refractory glaucoma.
Br J Ophthalmol 2003;87:63-70.



11. Choi YJ, Kim CS, Ahn BH. A comparison of the clinical effect between e-PTFE membrane-tube implant and Ahmed glaucoma valve implant for the treatment of refractory glaucoma.
Korean J Ophthalmol 2003;17:106-113.


12. Kim MK, Hwang YH, Ahn BH. Implantation of a modified baerveldt glaucoma implant with a smaller tube and intraluminal stent.
Korean J Ophthalmol 2017;31:90-91.



13. Bae HB, Kim CS, Ahn BH. A membranous drainage implant in glaucoma filtering surgery: animal trial.
Korean J Ophthalmol 1988;2:49-56.


14. Kim DW, Hwang YH, Ahn BH, et al. Safety and efficacy of a membrane-tube-type glaucoma shunt device: an animal trial.
Curr Eye Res 2017;42:890-896.


15. Sohn SW, Noh MD, Lee JH, et al. Performance of and pressure elevation formed by small-diameter microtubes used in constant-flow sets.
Korean J Ophthalmol 2016;30:225-233.



16. Francis BA, Cortes A, Chen J, Alvarado JA. Characteristics of glaucoma drainage implants during dynamic and steady-state flow conditions.
Ophthalmology 1998;105:1708-1714.


17. Rietveld E, van der Veen AJ AJ. Postoperative pressure regulation in glaucoma shunt surgery: focal tube constriction is not the answer.
J Glaucoma 2004;13:216-220.


18. Hoare Nairne JE JE, Sherwood D, Jacob JS, Rich WJ. Single stage insertion of the Molteno tube for glaucoma and modifications to reduce postoperative hypotony.
Br J Ophthalmol 1988;72:846-851.



19. Sheybani A, Reitsamer H, Ahmed II. Fluid dynamics of a novel micro-fistula implant for the surgical treatment of glaucoma.
Invest Ophthalmol Vis Sci 2015;56:4789-4795.


Fig.┬Ā1
A photograph of the perfusion apparatus and tubes (a black-colored box in the right inferior corner). To compare the tube diameters, a tube with a 200-┬Ąm external diameter and 100 ┬Ąm internal diameter with a 7-0 nylon intraluminal stent (left in the box) and a tube with a 300-┬Ąm external diameter and 200-┬Ąm internal diameter with a 5-0 nylon intraluminal stent (middle in the box) were placed together with a tube with a 630-┬Ąm external diameter and 300-┬Ąm internal diameter used for the conventional glaucoma drainage devices (right in the box).

Fig.┬Ā2
Schematic diagram of the perfusion apparatus used in the present experiment. The instrument consisted of a perfusion pump, syringe, pressure transducer, pressure detector, and a housing unit where the glaucoma implant was submerged in fluid. The height of the fluid was fixed at 3 cm (approximately 2.4 mmHg) from the plastic plate. The three-way stopcock was used to remove the fluid to maintain the baseline pressure during the experiments.
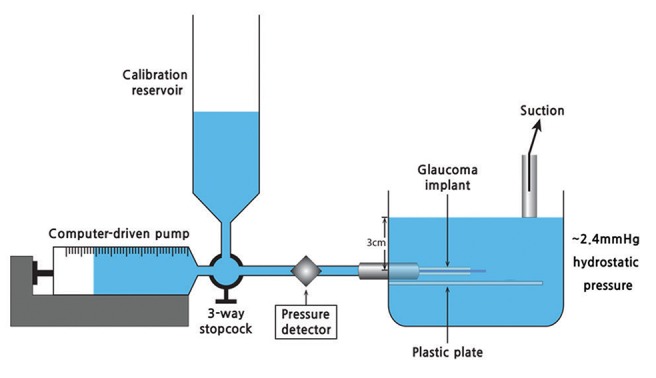
Fig.┬Ā3
Step function tests performed to measure the pressure according to each flow rate (2, 5, 10, and 25 ┬ĄL/min). Flow was maintained for up to 20 minutes at each step so that resistance was calculated from steady-state flow and pressure conditions.
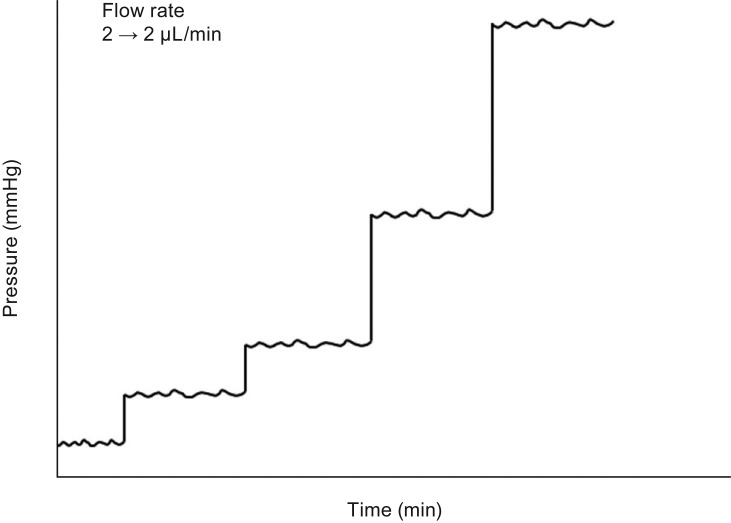
Fig.┬Ā4
Pressure variances in the tubes of membrane-tube (MT) type glaucoma shunt devices at each flow rate (2, 5, 10, and 25 ┬ĄL/min). (A) MicroMT and (B) Finetube MT. The pressure variances decreased according to the intraluminal stent retraction (p < 0.001 at all flow rates, Brown-Forsythe test).
Table┬Ā1
Flow, pressure, and resistance of the silicone tube of a MT type glaucoma shunt device (MicroMT) with and without an intraluminal stent (n = 30)
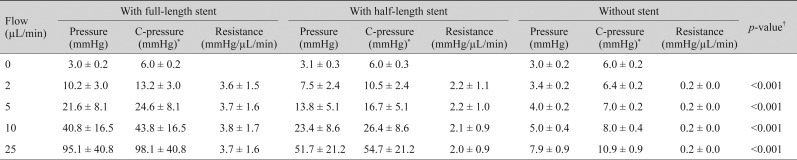
Table┬Ā2
Flow, pressure, and resistance of the silicone tube of a MT type glaucoma shunt device (Finetube MT) with and without an intraluminal stent (n = 30)












 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



