 |
 |
| Korean J Ophthalmol > Volume 33(3); 2019 > Article |
Abstract
Purpose
To evaluate the efficacy of anti-vascular endothelial growth factor (VEGF) treatment of eyes with foveal serous retinal detachment (SRD) associated with inferior staphyloma and to investigate choroidal thickness changes following anti-VEGF therapy.
Methods
In this observational case series, eyes with inferior staphyloma accompanied by foveal SRD were treated with a single intravitreal anti-VEGF injection, followed by further injections as needed. Changes in height and width of subretinal fluid (SRF) and visual acuity after treatment were assessed. Choroidal thickness was measured at the subfovea, 1.5 mm superior and inferior to the fovea using enhanced depth imaging optical coherence tomography at baseline and 1 month after initial anti-VEGF therapy.
Results
Six eyes from six patients were included. One month after the initial injection, the mean SRF height and width had decreased significantly from 112.5 ± 40.1 to 44.5 ± 48.7 µm (p = 0.046) and from 1,401.8 ± 627.3 to 690.7 ± 634.7 µm (p = 0.028), respectively. Mean choroidal thickness at the superior point decreased from 218.7 ± 59.3 to 200.5 ± 61.0 µm (p = 0.046). SRF resolved completely in three of the six eyes (50%) with a mean of 6.8 ± 5.9 injections (range, 1 to 15). All eyes experienced at least one recurrence of exudation, at a mean interval of 4.8 months. Mean visual acuity improvement was 0.17 logarithm of the minimum angle of resolution units at a mean of 28.7 months follow-up.
Inferior staphyloma is a type of primary posterior staphyloma associated with myopia; it was previously classified as a type V staphyloma [1]. This condition is sometimes accompanied by tilted disc syndrome, which is characterized by inferonasal tilting of the disc, peripapillary crescent, myopia, staphyloma of the affected inferonasal region, or thinning of the retinal pigment epithelium (RPE) at the inferonasal fundus [2]. Although the visual prognosis is good in most cases of inferior staphyloma, macular complications such as serous retinal detachment (SRD) occasionally develop and deteriorate visual function when the superior edge of the staphyloma traverses the macula [3,4,5,6].
Foveal SRD without neovascularization associated with inferior staphyloma rarely resolves spontaneously [7]. Moreover, very little is known about the treatment and prognosis of this kind of SRD. A previous study reported performing laser photocoagulation; however, the therapy was insufficient to ensure a favorable long-term visual outcome [4]. Despite the fact that the introduction of intravitreal anti-vascular endothelial growth factor (VEGF) therapy has revolutionized the treatment of choroidal neovascularization (CNV) secondary to age-related macular degeneration [8,9] and other conditions, such as myopia [10,11], recent studies have reported disappointing results of bevacizumab injection to treat foveal SRD in eyes with inferior staphyloma [12,13]. However, these previous reports involved only one or two cases, and it still remains unclear whether anti-VEGF therapy can improve clinical outcomes in eyes with inferior staphyloma accompanied by foveal SRD.
The use of enhanced depth imaging (EDI) optical coherence tomography (OCT) and swept source OCT have enabled better evaluation of the posterior choroid and sclera [14,15]. Using EDI-OCT, Yamagishi et al. [16] reported marked choroidal thinning at the upper border of the staphyloma. Ellabban et al. [17] showed relatively well-preserved choroid outside of the staphyloma and substantially thinned choroid within the staphyloma. Although studies have presented evidence of a possible association between morphological characteristics of choroid and foveal SRD, little is known about the effect of these characteristics on the outcome of anti-VEGF therapy.
Herein, we report the efficacy of anti-VEGF therapy for inferior staphyloma-related foveal SRD in six patients. Changes in choroidal thickness following anti-VEGF therapy were also examined.
This retrospective study comprised six eyes of six consecutive patients who had been diagnosed with foveal SRD without neovascular complications associated with inferior staphyloma, and were treated using intravitreal anti-VEGF at Seoul National University Hospital from July 2010 to October 2015. In all cases, the inferior staphyloma was lying across the macula and fluorescein angiography confirmed the absence of CNV. Eyes were excluded if they had a history of (1) intraocular surgery, conventional laser photocoagulation, or photodynamic therapy; (2) any type of CNV; (3) any other ocular pathology that could interfere with visual function (e.g., comorbid maculopathy); or (4) optical medial opacity that could significantly interfere with OCT image acquisition (e.g., cataract of more than Emery-Little classification grade III). The study was approved by the institutional review board of Seoul National University Hospital (1707-168-873) and adhered to the tenets of the Declaration of Helsinki. Approval for off-label use of bevacizumab was also obtained from the institutional review board committee. Informed consent was waived due to the retrospective nature of the study.
At baseline, a complete ocular examination was conducted on each patient, including best-corrected visual acuity (BCVA) measurement, tonometry, axial length measurement (Axis II PR; Quantel Medical, Bozeman, MT, USA), slit-lamp biomicroscopy, indirect fundus examination, fundus photography (Vx-10; Kowa Optimed, Tokyo, Japan), fluorescein angiography (FA, Vx-10), and spectral-domain OCT (Cirrus; Carl Zeiss Meditec, Dublin, CA, USA). At each follow-up visit, measurement of BCVA, slit-lamp biomicroscopy, dilated fundus examination, and OCT were performed. Measurements for BCVA were converted to logarithm of the minimum angle of resolution (logMAR) units for statistical analyses.
We performed spectral-domain OCT to obtain horizontal and vertical cross-sectional images of the macula using a 5-line raster scan mode with a length of 6 mm. Central foveal thickness (CFT), defined as the distance between the inner surface of the retina and the inner border of the RPE at the fovea, was measured using the built-in retinal mapping software of Cirrus OCT. The height of the subretinal fluid (SRF) at its thickest point (the distance between the lower interface of the detached retina and the upper interface of the RPE), as well as the largest diameter (width) of the SRF were measured manually using the OCT system's built-in calipers. Choroidal thickness was also measured manually with calipers as the distance from the outer edge of the RPE to the inner edge of the sclera on EDI-OCT images. These measurements were obtained at the subfovea, and 1.5 mm superior and inferior to the fovea on the vertical OCT lines passing through the fovea (Fig. 1).
Patients were administered a single 0.05 mL intravitreal injection of an anti-VEGF agent (1.25 mg bevacizumab [Avastin; Genentech, South San Francisco, CA, USA], 0.5 mg ranibizumab [Lucentis; Novartis, Basel, Switzerland], or 2.0 mg aflibercept [Eylea; Bayer HealthCare, Berlin, Germany]) at baseline. This was followed by pro re nata guided re-injections whenever recurrent or persistent SRF was detected on OCT. However, the decision to administer treatment was based on functional changes in vision as well as SRF changes. Therefore, no injection was performed if there were no functional changes in vision despite the presence of SRF. Under the sterile conditions of the outpatient surgical intervention room, intravitreal injections were administered via a 30-gauge needle through the pars plana at 3.5 mm posterior to the limbus. Recurrence was defined as the reappearance of SRF after complete SRF absorption, or any increase in SRF height. Recurrence interval was defined as the duration from the time of decreased or absorbed SRF to the time of re-increased SRF.
Primary outcomes, measured after anti-VEGF treatment, were resolution of SRF on OCT as well as changes in BCVA and choroidal thickness. The Wilcoxon signed rank test was used to compare mean CFT, height and width of the SRF, as well as choroidal thickness at baseline with those measured one month after the initial anti-VEGF injection. A p < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics ver. 21.0 (IBM Corp., Armonk, NY, USA).
Six eyes from six patients (two men [33.3%] and four women [66.7%]) with foveal SRD related to inferior staphyloma were included. Mean age was 55.3 ± 15.4 years (range, 36 to 76 years). Mean spherical equivalent refractive error was −5.59 ± 2.70 diopters (range, −9.13 to −3.25 diopters) and mean axial length was 25.74 ± 1.42 mm (range, 23.31 to 27.25 mm). Mean logMAR (Snellen) BCVA was 0.35 (20 / 44) ± 0.15 and mean CFT was 334.2 ± 32.0 µm (range, 284 to 375 µm).
Representative cases are illustrated in Fig. 2A-2H, 3A-3H, 4A-4F, 5A-5F, 6A-6G, 7A-7G. All six eyes showed depigmentation and thinning of the RPE in the inferior fundus, as well as SRD involving the center of the fovea. Five of the six eyes had tilted disc syndrome. In one of the six eyes, FA revealed band-shaped granular hyperfluorescence on the superior edge of the staphyloma; in another four eyes, FA demonstrated multiple pinpoint staining at the site of the RPE depigmentation. In the final eye, there was no evidence of leakage or staining on FA. In three eyes that underwent indocyanine green angiography, hypofluorescence during the early phase was noted on the border of the staphyloma. There were no lesions suggestive of CNV or polypoidal choroidal vasculopathy in the present study.
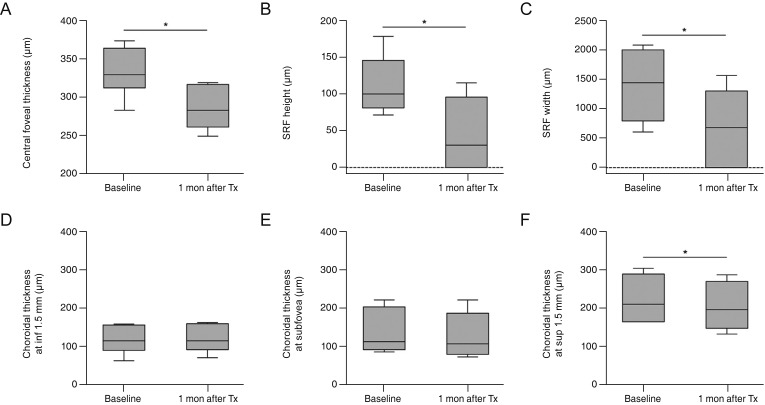
Box plots of the CFT, height and width of the SRF, and choroidal thickness in each region, both at baseline and one month after the initial anti-VEGF injection, are shown in Fig. 8. One month after anti-VEGF injection, the mean CFT had decreased from 334.2 ± 32.0 to 286.8 ± 29.9 µm, which was statistically significant (p = 0.028). Mean SRF height and width had also decreased significantly from 112.5 ± 40.1 to 44.5 ± 48.7 µm (p = 0.046), and from 1,401.8 ± 627.3 to 690.7 ± 634.7 µm (p = 0.028), respectively. One month after anti-VEGF injection, SRF had resolved completely in two of the six eyes (33.3%), while it was reduced but not completely resolved in the remaining four eyes (66.7%). At baseline, mean choroidal thicknesses on EDI-OCT vertical sections were 118.8 ± 36.2 µm at the point 1.5 mm inferior to the fovea, 135.7 ± 58.8 µm at the subfovea, and 218.7 ± 59.3 µm at the point 1.5 mm superior to the fovea. Mean choroidal thicknesses at both the subfovea (p = 0.028) and the inferior point (p = 0.027) were significantly less than that at the superior point. Furthermore, the mean choroidal thickness at the subfovea did not differ from that at the inferior point (p = 0.463). One month after anti-VEGF injection, mean choroidal thickness at the superior point had decreased to 200.5 ± 61.0 µm, which was statistically significant (p = 0.046). However, at both the subfovea and the inferior point, there was no significant change in choroidal thickness after therapy (p = 0.080 and p = 0.416, respectively).
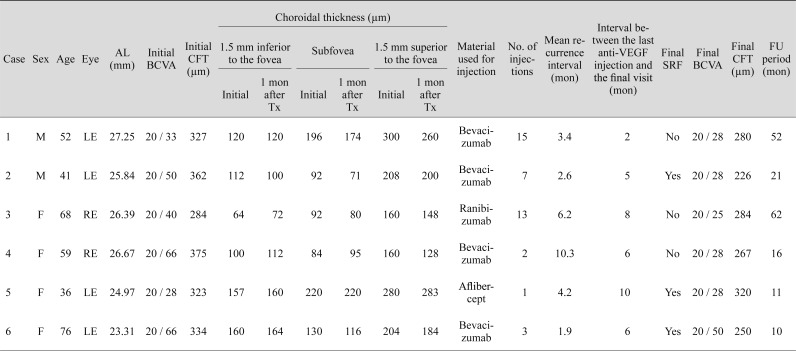
Treatment data and results are summarized in Table 1. Four eyes (66.7%) were administered bevacizumab, one (16.7%) was given ranibizumab, and one (16.7%) was injected with aflibercept. Mean number of injections was 6.8 ± 5.9 (range, 1 to 15). Four eyes (cases 1 to 4) have ever been achieved a dry macula during the follow-up period. During the course of therapy, SRF recurred at least once in all patients, at a mean of 4.8 ± 3.1 months (range, 1.9 to 10.3 months) following anti-VEGF treatment. At the final visit, SRF had completely resolved in three of the six eyes (cases 1, 3, and 4). Mean BCVA improved significantly from 0.35 (20 / 44) ± 0.15 logMAR at baseline to 0.19 (20 / 31) ± 0.11 logMAR after treatment at a mean of 28.7 ± 22.5 months follow-up (p = 0.043). Mean CFT had decreased significantly from 334.2 ± 32.0 to 271.2 ± 32.0 µm at the final visit (p = 0.043). There were no injection-related complications.
This study demonstrated the efficacy of anti-VEGF therapy in treating non-neovascular foveal SRD associated with inferior staphyloma. Anti-VEGF therapy resulted in a significant reduction in SRF and modest visual improvement in these patients. However, in most cases, recurrence necessitated multiple injections. To our knowledge, this is the first study to demonstrate a beneficial effect of anti-VEGF therapy on foveal SRD complicating inferior staphyloma.
The pathophysiology of SRD in inferior staphyloma remains controversial. Cohen et al. [7] suggested that the junctional area between the staphyloma and normal fundus corresponds to a region in which mechanical forces or hemodynamic changes occur. They hypothesized that choriocapillary and RPE disturbances at the junction of the inferior staphyloma permit subretinal leakage. Yamagishi et al. [16] reported marked choroidal thinning at the superior border of the staphyloma. They also speculated that SRD occurs as a result of a decreased ability of the choroid to remove SRF. Furthermore, hypofluorescence often occurred during the early phase of indocyanine green angiography in eyes with inferior staphyloma; this was likely due to atrophy of the choriocapillaris and marked subfoveal choroidal thinning. Consequently, atrophy of the choriocapillaris and impairment of the microvascular circulation due to choroidal thinning may cause focal hypoxic damage. Subsequent focal choroidal ischemia may upregulate VEGF expression and VEGF-induced vascular leakage. Meanwhile, Maruko et al. [18] showed choroidal thinning and scleral thickening at the fovea; they suggested that choroidal fluid within the inferior staphyloma might not pass through the thickened sclera, and that it may instead leak into the subretinal space through a degenerated RPE. In all our patients, the site of leakage was the edge of the staphyloma in an area of RPE change. In addition, the subfoveal choroid, which generally coincides with the superior border of the staphyloma, was significantly thinned in all eyes. However, we were not able to determine the outer border of the sclera in any of our patients, even with EDI-OCT; therefore, we could not measure scleral thickness.
Although the exact mechanism of SRF absorption after anti-VEGF therapy was not determined in our study, we hypothesize that the therapy prevents VEGF-induced vascular leakage, as VEGF alters tight junctions and promotes vascular permeability in many retinal diseases [19,20]. Anti-VEGF therapy may therefore reduce choriocapillaris fenestrations and restore RPE tight junctions. Decreased choroidal permeability that may have resulted from reduction in VEGF concentration following the anti-VEGF therapy may have contributed to the decreased choroidal thickness at the point 1.5 mm superior to the fovea in this study. However, at both the subfovea and the inferior point, no significant changes in choroidal thickness were observed after therapy, presumably due to a floor effect. In other words, those with a markedly thinned choroid at baseline had less room for a further decrease in thickness after treatment. Based on our findings, we suggest that anti-VEGF therapy may affect the choroidal vasculature, and therefore play a role in the resorption of SRF in eyes with inferior staphyloma. On the other hand, there is also concern that repeated injections of anti-VEGF agents may accelerate hypoxic changes and RPE damage, which could lead to persistent SRD in the future. Saenz-de-Viteri et al. [21] analyzed the effects of single or repeated doses of anti-VEGF agents on ARPE-19 cells, an adult human RPE cell line that shows many differentiated properties of RPE cells in vivo. They found that three anti-VEGF drugs at doses used in clinical practice were not significantly toxic to RPE cells in vitro, even when cells were treated repeatedly. Moreover, they reported that anti-VEGF agents could have a preventive effect on maintenance of the RPE barrier under oxidative stress conditions. Nevertheless, more studies are needed to accurately evaluate the effect of anti-VEGF agents on RPE cells.
Our current finding—namely that anti-VEGF therapy had a beneficial effect in eyes with inferior staphyloma-related foveal SRD—is inconsistent with those of previous studies, which have reported no effect. Milani et al. [12] reported that one patient who was affected by macular SRD associated with tilted disc syndrome did not benefit from two consecutive monthly injections of bevacizumab. Similarly, Donati et al. [13] described two patients with macular SRD associated with tilted disc syndrome, one of whom was treated using three injections of bevacizumab monotherapy, while the other was treated using combined bevacizumab and photodynamic therapy. Treatment was unsuccessful in both patients. It may be that in these previous studies, fewer than necessary anti-VEGF injections were administered, resulting in the discrepancy with our own findings. In case 2 of the current study, SRF initially decreased after treatment, but persisted after three consecutive bevacizumab injections (Fig. 3); after three more bevacizumab injections, the SRF resolved completely. Recently, Hirano et al. [22] reported one case of SRD in inferior staphyloma that was refractory to ranibizumab treatment, but in which exudative changes resolved after two injections of aflibercept.
This study had several limitations. Firstly, the number of cases was limited, and the follow-up period was short for some cases. Further studies with more patients and a longer follow-up duration are required to confirm our results. The lack of a control group is another important limitation of this study. Indeed, it is possible that some of our cases resolved spontaneously, and that the resolution was simply coincident with the treatment. An additional limitation is that, due to the small number of cases, we could not analyze choroidal thickness in subgroups separated on the basis of SRF resolution 1 month after the initial anti-VEGF injection. Additional studies evaluating such subgroups are necessary to further elucidate the pathogenesis of this disease and develop a better understanding of how intravitreal anti-VEGF therapy confers its beneficial effects.
In conclusion, anti-VEGF therapy resulted in a significant decrease in SRF and a modest visual improvement in eyes with foveal SRD associated with inferior staphyloma. Reduction of the superior choroidal thickness might be the therapeutic mechanism of anti-VEGF treatment. Frequent recurrences and insufficient responses in some patients, however, still need to be addressed.
Acknowledgements
This work was supported in part by the research grant from the Korean Association of Retinal Degeneration.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
REFERENCES
2. Apple DJ, Rabb MF, Walsh PM. Congenital anomalies of the optic disc. Surv Ophthalmol 1982;27:3-41.


4. Leys AM, Cohen SY. Subretinal leakage in myopic eyes with a posterior staphyloma or tilted disk syndrome. Retina 2002;22:659-665.


5. Theodossiadis PG, Grigoropoulos V, Emfietzoglou J, Theodossiadis GP. Optical coherence tomography study of tilted optic disk associated with macular detachment. Graefes Arch Clin Exp Ophthalmol 2006;244:122-124.


6. Nakanishi H, Tsujikawa A, Gotoh N, et al. Macular complications on the border of an inferior staphyloma associated with tilted disc syndrome. Retina 2008;28:1493-1501.


7. Cohen SY, Quentel G, Guiberteau B, et al. Macular serous retinal detachment caused by subretinal leakage in tilted disc syndrome. Ophthalmology 1998;105:1831-1834.


8. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006;355:1432-1444.


9. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419-1431.


10. Ikuno Y, Sayanagi K, Soga K, et al. Intravitreal bevacizumab for choroidal neovascularization attributable to pathological myopia: one-year results. Am J Ophthalmol 2009;147:94-100.


11. Silva RM, Ruiz-Moreno JM, Rosa P, et al. Intravitreal ranibizumab for myopic choroidal neovascularization: 12-month results. Retina 2010;30:407-412.


12. Milani P, Pece A, Pierro L, et al. Bevacizumab for macular serous neuroretinal detachment in tilted disk syndrome. J Ophthalmol 2010;2010:970580.



13. Donati MC, Miele A, Abbruzzese G, et al. Treatment of macular serous neuroretinal detachment in tilted disk syndrome: report of 3 cases. Eur J Ophthalmol 2013;23:267-270.


14. Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol 2008;146:496-500.


15. Hirata M, Tsujikawa A, Matsumoto A, et al. Macular choroidal thickness and volume in normal subjects measured by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci 2011;52:4971-4978.


16. Yamagishi T, Koizumi H, Yamazaki T, Kinoshita S. Choroidal thickness in inferior staphyloma associated with posterior serous retinal detachment. Retina 2012;32:1237-1242.


17. Ellabban AA, Tsujikawa A, Matsumoto A, et al. Macular choroidal thickness measured by swept source optical coherence tomography in eyes with inferior posterior staphyloma. Invest Ophthalmol Vis Sci 2012;53:7735-7745.


18. Maruko I, Iida T, Sugano Y, et al. Morphologic choroidal and scleral changes at the macula in tilted disc syndrome with staphyloma using optical coherence tomography. Invest Ophthalmol Vis Sci 2011;52:8763-8768.


19. Murakami T, Felinski EA, Antonetti DA. Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J Biol Chem 2009;284:21036-21046.



20. Nicoletti VG, Nicoletti R, Ferrara N, et al. Diabetic patients and retinal proliferation: an evaluation of the role of vascular endothelial growth factor (VEGF). Exp Clin Endocrinol Diabetes 2003;111:209-214.


Fig. 1
An enhanced depth imaging optical coherence tomography image demonstrating the morphologic parameters measured in this study. The dotted line indicates the posterior edge of the choroid, and the white double arrows indicate the choroidal thickness. SRF = subretinal fluid.

Fig. 2
A 52-year-old man (case 1) with foveal serous retinal detachment associated with inferior staphyloma. (A) Fundus photograph revealed inferior staphyloma and inferior peripapillary crescent (arrow). (B) Fluorescein angiography showed a belt-shaped area of granular hyperfluorescence (arrowheads) corresponding to the border of the inferior staphyloma. (C) Indocyanine green angiography demonstrated a hypofluorescent band (arrowheads). (D) Baseline spectral-domain optical coherence tomography showed foveal serous retinal detachment. (E) After two bevacizumab injections, subretinal fluid (SRF) resolved completely. (F) The patient experienced a mild recurrence and resolution after the injection, and he received four bevacizumab injections over a period of 2 years. Thereafter, SRF recurred. (G) After two more injections, SRF decreased. (H) Seven additional bevacizumab injections were performed with repeated recurrence and resolution, and no SRF was observed at the last visit.
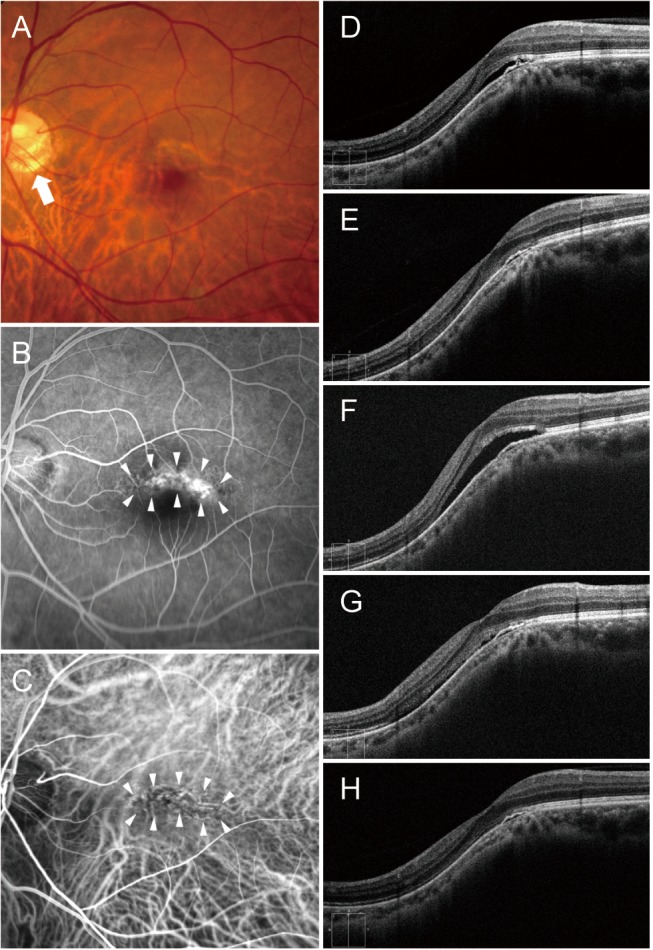
Fig. 3
A 41-year-old man (case 2) with foveal serous retinal detachment (SRD) associated with inferior staphyloma. (A) Fundus photograph showed inferior staphyloma with a superior border across the macula. (B) Fluorescein angiography revealed multiple pinpoint staining (arrowheads). (C) Indocyanine green angiography demonstrated subtle hypofluorescence (arrowheads). (D) Baseline spectral-domain optical coherence tomography showed foveal SRD. (E) After three bevacizumab injections, subretinal fluid (SRF) decreased slightly, but persisted. (F) Complete resolution of SRF was noted after three more bevacizumab injections. (G) SRD recurred 3 months later. (H) After one more injection, SRF decreased, but remained present.
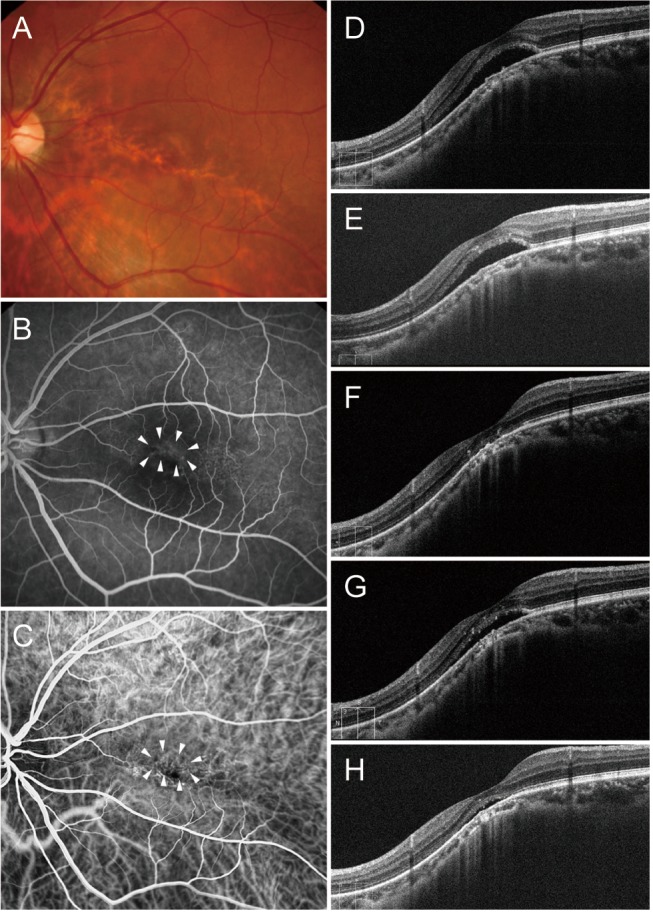
Fig. 4
A 68-year-old woman (case 3) with foveal serous retinal detachment associated with inferior staphyloma. (A) Fundus photograph showed inferior staphyloma and inferior peripapillary crescent (arrow). (B) Fluorescein angiography revealed multiple pinpoint staining (arrowheads). (C) Baseline spectral-domain optical coherence tomography demonstrated foveal serous retinal detachment. (D) After one ranibizumab injection, the subretinal fluid (SRF) resolved completely. (E) The patient experienced repeated mild recurrence and resolution after the injection, and she received nine ranibizumab injections over a period of 3 years. Thereafter, a small amount of SRF accumulated again. (F) After two additional injections for 8 months, no SRF was observed at the last visit.

Fig. 5
A 59-year-old woman (case 4) with foveal serous retinal detachment associated with inferior staphyloma. (A) Fundus photograph showed inferior staphyloma and inferior peripapillary crescent (arrow). (B) Fluorescein angiography revealed multiple pinpoint staining (arrowheads). (C) Baseline spectral-domain optical coherence tomography demonstrated foveal serous retinal detachment. (D) After one bevacizumab injection, subretinal fluid (SRF) resolved completely. (E) SRF increased 10 months later. (F) After one more bevacizumab injection, the SRF was completely absorbed.
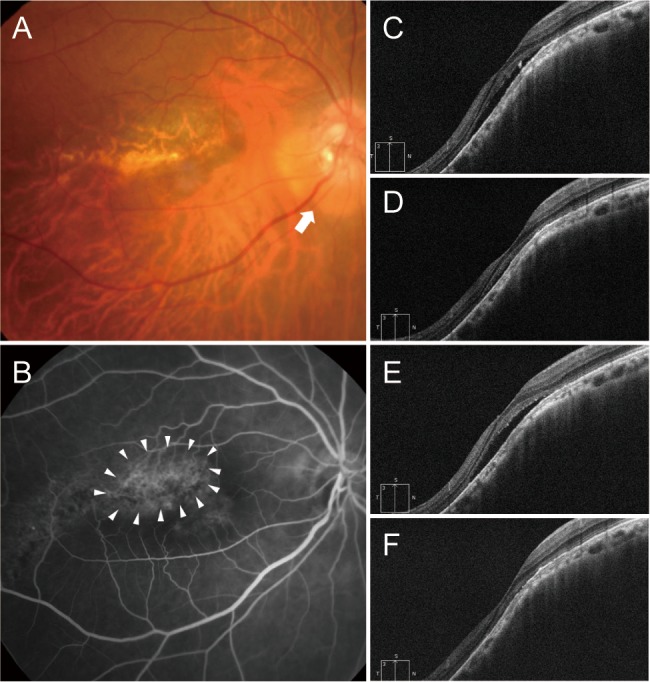
Fig. 6
A 36-year-old man (case 5) with foveal serous retinal detachment (SRD) associated with inferior staphyloma. (A) Fundus photograph showed inferior staphyloma and inferior peripapillary crescent (arrow). (B) There was no evidence of leakage or staining on fluorescein angiography. (C) Indocyanine green angiography demonstrated subtle hypofluorescence (arrowheads). (D) Baseline spectral-domain optical coherence tomography showed foveal SRD. (E) One aflibercept injection decreased the amount of subretinal fluid (SRF) slightly, but the SRF persisted. (F) The SRF had increased 4 months later. (G) The patient declined further injections. At the final visit, the foveal SRD remained.
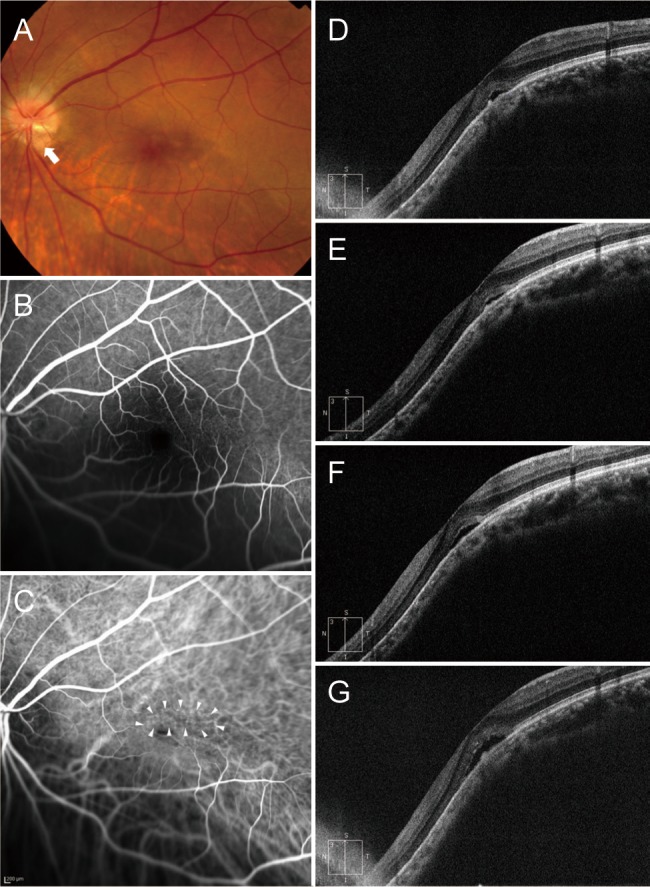
Fig. 7
A 76-year-old woman (case 6) with foveal serous retinal detachment associated with inferior staphyloma. (A) Fundus photograph showed inferior staphyloma and inferior peripapillary crescent (arrow). (B) Fluorescein angiography revealed multiple pinpoint staining (arrowheads). (C) Baseline spectral-domain optical coherence tomography showed foveal serous retinal detachment. (D) Two bevacizumab injections reduced the amount of subretinal fluid (SRF), but SRF was still present. (E) SRF had increased 2 months later. (F) After one more bevacizumab injection, SRF decreased slightly, but remained. (G) The patient declined further injections. At the final visit, little SRF remained.

Fig. 8
Comparison of measurements at baseline and 1 month after the initial anti-vascular endothelial growth factor injection. (A) Central foveal thickness, (B) height and (C) width of subretinal fluid (SRF), and (D) choroidal thicknesses measured 1.5 mm inferior to the fovea, (E) at the subfovea, and (F) 1.5 mm superior to the fovea. Box plots indicate median values with 5th and 95th percentiles. Tx = treatment. *p < 0.05 by Wilcoxon signed rank test.
- TOOLS
-
METRICS

-
- 4 Crossref
- 0 Scopus
- 2,583 View
- 24 Download
- Related articles



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



