Dear Editor,
The Baerveldt glaucoma implant (BGI; Abbott Laboratories, Abbott Park, IL, USA) is a widely used glaucoma drainage device for intraocular pressure (IOP) control. Although the BGI effectively controls IOP, the possibility of postoperative ocular hypotony owing to excessive aqueous drainage and tube-related complications still remains [1,2]. Given that the a mount of a queous drainage at the early postoperative period is mainly determined by tube profiles, we reasoned that a tube with a smaller diameter and intraluminal stent may have a lower risk of postoperative ocular hypotony [3,4,5]. In addition, a smaller tube may have a lower chance of tube-related complications, such as conjunctival erosion or tube exposure [3,4,5]. To test this, we replaced the conventional silicone tube of the BGI (630-┬Ąm external and 300-┬Ąm internal diameter) with a smaller silicone tube (300-┬Ąm external and 200-┬Ąm internal diameter) and an intraluminal stent (5-0 nylon); we named this tube ŌĆ£Fine-tube [4].ŌĆØ We implanted the modified BGI with a Finetube into three eyes from three patients with uncontrolled IOP (36, 34, and, 30 mmHg, respectively) despite previous trabeculectomies, as well as medical treatment.
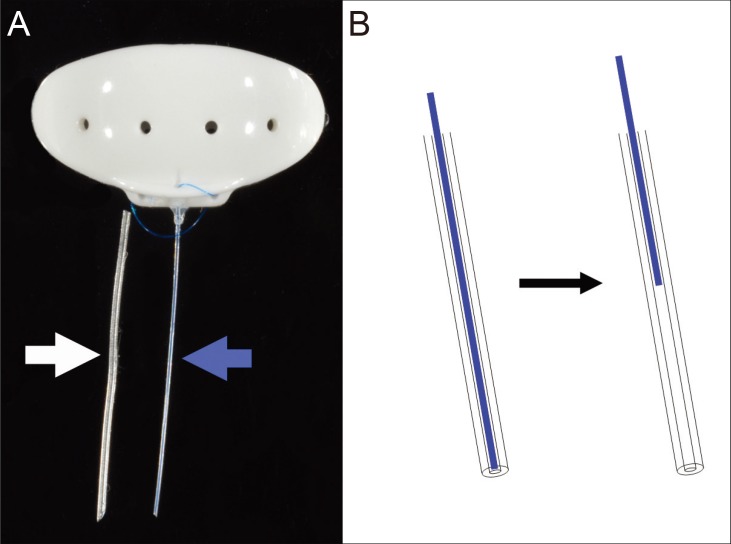
Preoperatively, the conventional BGI tube was cut approximately 1 mm from the body plate. The Finetube was inserted into the stump of the conventional tube and fixed using silicone adhesive (Fig. 1A). An intraluminal stent prevents excessive aqueous drainage through the tube, and induces additional IOP reduction after surgery by retracting the stent (Fig. 1B). The main conjunctival incision was made 8 mm posterior to the limbus and the BGI body plate was placed under the superior and lateral recti muscles and fixed to the bared sclera, 10 mm posterior to the limbus. The second conjunctival incision was made 2 mm posterior to the limbus. A scleral tunnel, from the limbal second incision to the forniceal main incision was made using a 23-gauge needle. The needle tip was used to guide the Finetube towards the second incision. After the tube was trimmed to an appropriate length, it was inserted into the anterior chamber through the corneoscleral track created using a 26-gauge needle as described previously [4]. The corneoscleral track and conjunctiva were closed using 10-0 nylon sutures. A part of the 5-0 nylon stent was left partially exposed through the conjunctiva to be pulled out when the IOP needed to be further reduced [4].
At the early postoperative period (within 1 month), the IOP ranged from 6 to 15 mmHg; no eye showed postoperative ocular hypotony. Even though the IOP was not high, the intraluminal stents were completely removed in all cases 4 weeks after surgery when flow restriction by the stent for the prevention of postoperative ocular hypotony was no longer needed [4]. IOP was successfully controlled (8 to 17 mmHg) for 18, 34, and 36 months, respectively. There were no complications, such as postoperative ocular hypotony and tube-related complications (conjunctival erosion, tube exposure, migration, infection, and occlusion). These findings implied that a smaller tube may induce an effective IOP reduction with a low chance of complications which are in line with previous study results [3,4].
Given that aqueous drainage through the tube is mainly determined by the tube diameter, a modified BGI with a Finetube may have a lower chance of postoperative ocular hypotony compared to conventional BGI. An intraluminal stent can control the amount of aqueous drainage through the tube lumen after surgeryŌĆöthe stent can be retracted to increase drainage if necessary. Therefore, a safe, predictable, and gradual IOP reduction can be achieved [3,4,5]. In addition, because a smaller tube has a less volume, a modified BGI with a Finetube may cause less conjunctival erosion and tube exposure than conventional BGI. A possible shortcoming of a smaller tube is tube occlusion by inflammatory materials, blood clots, or silicone oil [4]. In this case series, no eye showed tube occlusion. However, further studies regarding this issue are needed.
In our cases series, a modified BGI with a Finetube showed effective and safe IOP control without ocular hypotony and tube-related complications. Further studies with a larger number of subjects would be necessary.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print






