Ophthalmologic Features of Lennox-Gastaut Syndrome
Article information
Abstract
Purpose
To describe the characteristics and frequency of ophthalmologic findings in patients with Lennox-Gastaut syndrome (LGS).
Methods
The medical records of patients diagnosed with LGS at Seoul National University Children's Hospital from January 2004 to August 2014 were retrospectively reviewed. The records of 34 patients (mean age ± standard deviation, 2.66 ± 3.51 years; male, 58.8%) were reviewed. The primary measure was the incidence of ophthalmologic manifestations.
Results
Of the 34 patients, 88.2% had at least one ocular abnormality. Refractive error (52.9%) was the most frequently observed ophthalmologic manifestation in patients with LGS, followed by strabismus (32.4%), cortical visual impairment (23.5%), and retinopathy of prematurity (8.8%). Among these cases, seven patients had exotropia and three had esotropia.
Conclusions
LGS is a childhood-onset epileptic encephalopathy with variable ophthalmologic manifestations, the most frequent being refractive errors. Patients with suspected LGS should be examined regularly because ophthalmological features can change during their disease course.
Lennox-Gastaut syndrome (LGS) is a childhood epileptic encephalopathy characterized by an electroclinical triad of generalized slow spike and wave complex of 1.5 to 2.5 Hz on electroencephalogram, multiple types of seizures such as atypical absence and tonic seizures, and behavioral disorders including cognitive development disorder [1]. LGS accounts for approximately 1% to 5% of all pediatric epilepsies and its onset occurs between 1 and 8 years of age with the first epileptic episode, most commonly occurring between 3 and 5 years of age [2]. Approximately 20% to 30% of patients with this syndrome show normal development and no indications of organic brain dysfunction in various tests prior to disease onset. Common structural or metabolic causes of LGS include congenital brain malformation, hypoxic-ischemic brain injury, encephalitis, meningitis, and nodular sclerosis. Focal brain lesions, especially lesions in the frontal lobe, may be associated with this syndrome [3].
Although the pathogenesis of this syndrome has not been firmly established, since it is a developmental disorder involving the central nervous system, it may be accompanied by abnormalities in the visual or ocular motor system. To the best of our knowledge, no studies have investigated the ophthalmologic features that accompany this disease. Therefore, in the present study, we aimed to examine the characteristics and frequency of ophthalmologic features in patients with LGS.
Materials and Methods
Of the patients who were clinically diagnosed with LGS in the Department of Pediatrics at Seoul National University Children's Hospital between January 2004 and August 2014, only patients who underwent ophthalmologic consultation were included in the present study. Diagnostic criterion for LGS was set as having three disease-specific findings of slow spike and wave complex on electroencephalogram, multiple types of seizures, and cognitive development disorder.
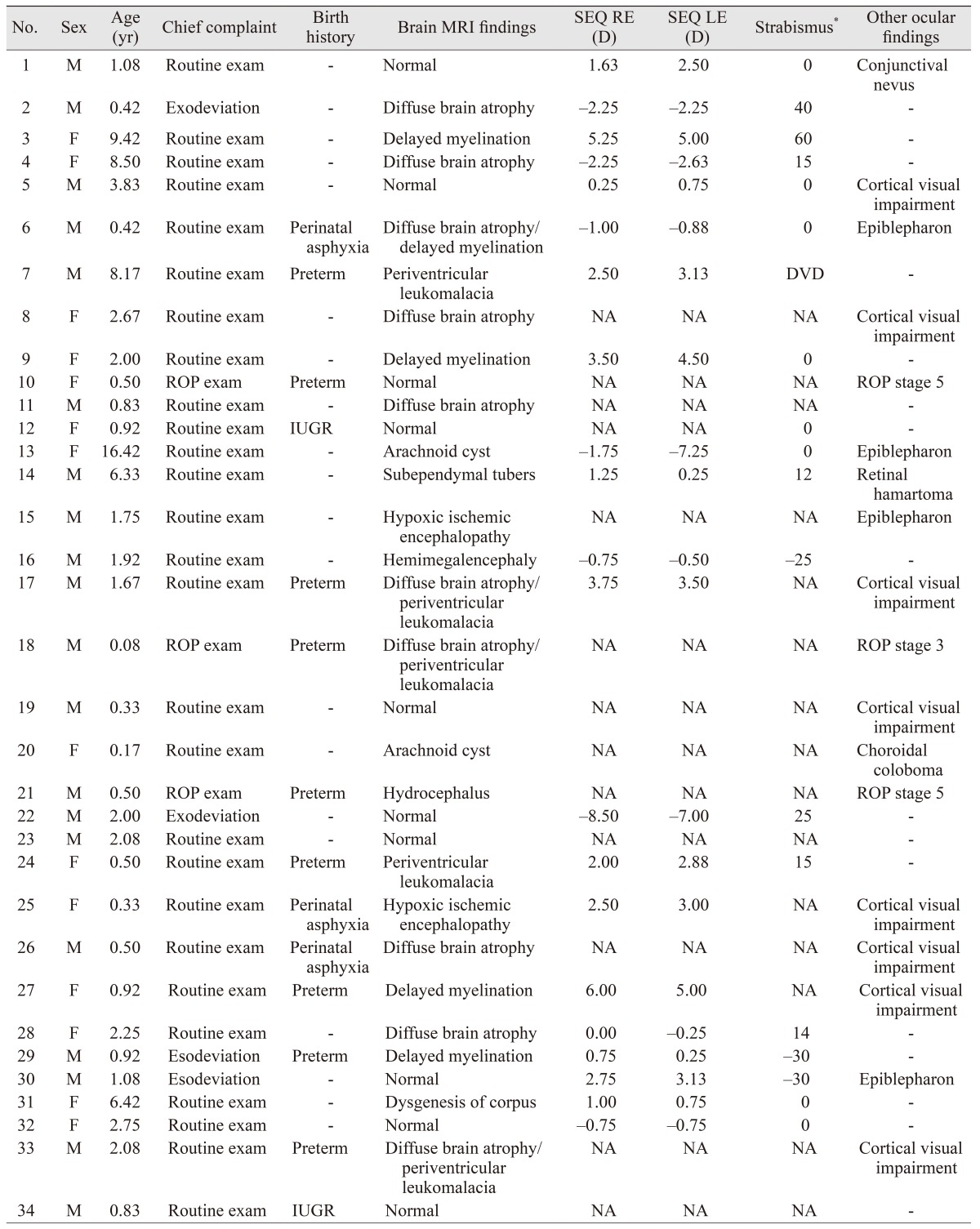
Based on a retrospective review of medical records, among 303 patients with LGS, 34 patients who underwent ophthalmologic examination were included in this study. Medical and birth histories, as well as brain magnetic resonance imaging (MRI) from the patients' medical records were analyzed. Brain MRI was performed on all patients. The ophthalmic evaluations included slit-lamp biomicroscopy, fundus examinations, refraction test, and measurement of angle of deviation.
To determine the degree of refractive error in patients, a cycloplegic refraction test was performed on all patients. Refractive error was defined as spherical equivalent ≤−1.00 diopters (D) for myopia and ≥+2.00 D for hyperopia, while astigmatism was defined as a cylindrical refractive error ≥1.50 D [4].
For suspected cases of strabismus, the angle of deviation was measured via the alternate prism cover test, when cooperation was possible, and via the Hirschberg test or Krimsky prism test, when cooperation was not possible. Strabismus was defined as constant or intermittent heterotropia of any magnitude at distance or near fixation. Strabismus was classified according to the horizontal direction (esotropia or exotropia) of the tropia or as vertical strabismus if there was no horizontal tropia [5].
In cases where the visual acuity was too poor to fix and follow, refraction, fundus, and pupil response examinations were used to verify that there were no anomalies in the eyes or the visual pathway. Those who did not generate a waveform in the visual evoked potential test and showed visual cortex damage on brain MRI were diagnosed with cortical visual impairment.
Results
The patients included 20 males and 14 females; mean age was 2.66 ± 3.51 years and the mode age was 0.50 years (1 month to 16 years). Birth history revealed nine cases (26.5%) of preterm birth, three cases (8.8%) of perinatal asphyxia, and two cases (5.9%) of intrauterine growth retardation (IUGR) (Table 1). Among all patients, 30 patients (88.2%) were found to have at least one ophthalmologic abnormality. The most common ophthalmologic findings were refractive errors (n = 18, 52.9%), including 13 patients with astigmatism (38.2%), nine with hyperopia (26.5%), and five with myopia (14.7%). Astigmatism, hyperopia, and myopia were observed in the ranges of 1.50 D to 5.00 D, +2.00 D to +6.00 D, and −1.00 D to −8.50 D, respectively.
Strabismus was found in 11 patients (32.4%), with seven having exotropia (20.6%), three having esotropia (8.8%), and one having dissociated vertical deviation (2.9%). Exotropia and esotropia were observed in the ranges of 12 to 60 prism diopters (PD) and −25 to −30 PD, respectively. In addition, eight patients (23.5%) showed cortical visual impairment.
History of retinopathy of prematurity was found in three patients (8.8%), all of whom had previously undergone peripheral retinal laser photocoagulation, showing scarring on the peripheral retina.
No patients showed abnormal findings, including cataract, in the slit-lamp examination. Except for the three children with a history of retinopathy of prematurity, one child with retinal hamartoma, and one child with choroidal coloboma, no other retinal abnormalities were found.
Brain MRI was performed on all subjects, and revealed brain parenchymal atrophy in 10 patients (29.4%), delayed myelination in five patients (14.7%), and periventricular leukomalacia in another five patients (14.7%).
Discussion
LGS is characterized by a number of different clinical neurological features appearing mostly during childhood. Although its pathogenesis has not been firmly established, congenital brain malformation, hypoxic-ischemic brain injury, encephalitis, meningitis, and nodular sclerosis have been associated with the disease and they can manifest as various neurological features [3]. Moreover, ophthalmological symptoms can also occur. However, there have been no reports on the actual ophthalmologic features of LGS.
Little is known about the frequency of ophthalmologic symptoms or signs related to LGS, and the present study investigated the frequency of ophthalmologic findings in patients with LGS. Refractive errors were observed most frequently in patients with LGS, and in addition, strabismus and cortical visual impairment were also observed.
Refractive errors consisted of 13 cases (38.2%) of astigmatism, nine cases (26.5%) of hyperopia, and five cases (14.7%) of myopia, all of which were relatively higher than the rates of astigmatism, hyperopia, and myopia of 8.29%, 13.47%, and 3.98%, respectively, as observed in children in Asian countries [4].
The present study also found 11 patients (32.4%) with strabismus compared to the 3.55% strabismus rate seen in children in Asian countries, demonstrating that strabismus occurred at a markedly higher rate in patients with LGS [5]. Various perinatal issues, such as preterm birth, low birth weight, and congenital malformations are known risk factors of strabismus, and in particular, IUGR is known to be a risk factor for exotropia [67]. We postulate that since 41.2% of patients in the present study had some form of perinatal complication, such as preterm birth and IUGR, this may have contributed to the higher rate of strabismus observed in this study.
In addition, cortical visual impairment was observed in eight patients (23.5%). Localized or multiple abnormalities in cerebral cortical development are commonly seen in LGS [3], and thus, abnormalities of the visual cortex could lead to cortical visual impairment.
Previous studies have reported on genetic anomalies that concurrently manifest with epileptic encephalopathy and strabismus [89], and therefore, ophthalmologic features that appear in LGS can be approached from this perspective.
The present study had the limitation of being a retrospective study with a small number of subjects. Moreover, the subjects were confined to patients with LGS who had been referred for ophthalmologic examinations rather than all patients with LGS, which could result in selection bias. Although the prevalence of each ophthalmologic abnormality might appear higher than the actual value because of this bias, the present study was significant in that it was the first to analyze the ophthalmologic features and their distributions in patients with LGS.
Furthermore, we believe that even for patients with LGS who do not show distinct ophthalmologic symptoms, the findings of the present study can be applied for early detection of ophthalmologic disorders through regular ophthalmologic examination to assist in developing visual acuity and binocular vision. Therefore, future studies with a greater number of patients are warranted.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.