 |
 |
| Korean J Ophthalmol > Volume 31(3); 2017 > Article |
Abstract
Purpose
To evaluate the 12-month outcome of intravitreal anti-vascular endothelial growth factor therapy in eyes with bilateral retinal angiomatous proliferation (RAP).
Methods
This retrospective observational study included 38 eyes of 19 patients with stage 1 or 2 bilateral RAP at diagnosis. The eyes of patients who exhibited different baseline best-corrected visual acuity (BCVA) values in both eyes were assigned to one of two groups—the better (n=13) and worse (n=13) visual acuity groups. The BCVA values in both groups were compared to those at baseline and at 12 months. In addition, the 12-month changes in BCVA were compared between the two groups. The association between the optical coherence tomography findings at diagnosis and the 12-month BCVA was also analyzed.
Results
The values of mean baseline and 12-month BCVA in the better visual acuity group (13 eyes) were 0.48 ± 0.19 and 0.58 ± 0.29, respectively, and those in the worse visual acuity group (13 eyes) were 0.83 ± 0.20 and 0.90 ± 0.31. The 12-month changes in BCVA were not significantly different between the two groups (p=0.786). Among the six patients with equivalent baseline BCVA in both eyes, four patients (66.7%) exhibited 1 to 2 lines or ≥3 lines of difference in BCVA between eyes at 12 months. Eyes without pigment epithelial detachment (PED) at diagnosis exhibited significantly better BCVA at 12 months than eyes with PED (p=0.021).
Conclusions
Better baseline visual acuity was associated with better BCVA at 12 months posttreatment in patients with bilateral RAP. However, equivalent baseline visual acuity in both eyes might not guarantee similar treatment outcomes. In addition, the absence of PED is predictive of better visual outcome.
Retinal angiomatous proliferation (RAP) is a subtype of exudative age-related macular degeneration (AMD) that is characterized by retina-retinal or retinal-choroidal anastomosis [1]. One of the characteristics of RAP, which distinguishes it from other types of exudative AMD, is the relatively high risk of bilateral involvement. In a previous study involving 108 patients, Yannuzzi et al. [1] reported bilateral RAP at diagnosis in 26 patients. The incidence of fellow-eye involvement in patients with unilateral RAP is reported to be between 27% and 100% [2,3,4,5].
Anti-vascular endothelial growth factor (VEGF) is an effective treatment for exudative AMD [6,7,8,9,10], and previous studies have also demonstrated the efficacy of this therapy in RAP [11,12,13,14,15,16,17,18,19]. However, to the best of our knowledge, the outcome of bilateral RAP treatment with anti-VEGF has not yet been reported in detail, despite the fact that bilateral involvement at diagnosis is a frequent finding in patients with RAP. The purpose of this study was to evaluate the 12-month outcome of intravitreal anti-VEGF therapy in eyes with recently-developed bilateral RAP.
This single-center observational case study followed the tenets of the Declaration of Helsinki and was approved by the institutional review board of Kim's Eye Hospital (Seoul, Korea). Written informed consent from the patients was not required for this retrospective study. All patient records/data were anonymized and de-identified prior to analysis.
We reviewed the medical records of patients diagnosed with RAP between January 2010 and December 2012 and included patients who were treated with anti-VEGF monotherapy and underwent follow-up for at least 12 months. All of the included patients were subjected to comprehensive ophthalmological examination, including measurement of best-corrected visual acuity (BCVA), 90-diopter (D) lens slit-lamp biomicroscopy, and fundus photography. Infrared images of the retina were acquired by spectral-domain optical coherence tomography (OCT; Spectral OCT/SLO, OTI Ophthalmic Technologies, Miami, FL, USA). Fundus autofluorescence, indocyanine green angiography, and red-free images were acquired using a confocal laser-scanning system (HRA-2; Heidelberg Engineering, Dossenheim, Germany).
The exclusion criteria were as follows: duration of symptoms greater than 6 months or presence of stage 3 RAP (chorioretinal anastomosis) in either eye, severe media opacity, end-stage AMD (central geographic atrophy and disciform scarring), history of intraocular surgery (except cataract surgery), macroaneurysms, proliferative diabetic retinopathy, central retinal vascular occlusions, and any other retinal disorder that might influence the macular microstructure and/or function. Eyes with submacular hemorrhages ≥1 disc diameter and that involved the fovea at the time of diagnosis were also excluded.
All included eyes were treated with three consecutive monthly intravitreal ranibizumab injections as initial treatment. Injections for both eyes were administered on the same day or within 1 week of each other. The patients were examined every 1 to 3 months thereafter, as determined by the treating physician. In patients without recurrence for relatively long periods, the follow-up interval was extended to 3 months. At the follow-up examinations, the patients were evaluated by fluorescein angiography or indocyanine green angiography, at the discretion of the clinician. Patients with persistent intraretinal/subretinal fluid after initial treatment or recurrence were treated again with an intravitreal anti-VEGF agent (ranibizumab or bevacizumab). Recurrence was defined by visualization of reaccumulated subretinal or intraretinal fluid in the OCT images following initial treatment, after the fluid had completely resolved. The visualization of newly-developed retinal/subretinal hemorrhage upon clinical examination was also considered recurrence.
Reticular pseudodrusen was diagnosed based on multimodal imaging findings. Reticular pseudodrusen was diagnosed when the condition was confirmed by one or more of the following examinations: color fundus photography, blue-channel image fundus photography, red-free imaging, infrared imaging, or fundus autofluorescence imaging. Blue-channel fundus images were obtained using an image editing program (Photoshop CS4; Adobe Systems, San Jose, CA, USA). In some cases, the contrast of the color fundus photographs was enhanced using Photoshop CS4 for better visualization. Central foveal thickness (CFT) was manually measured from the OCT images using the built-in calipers of the OCT software and was defined as the distance between the internal limiting membrane and the Bruch's membrane at the fovea. The results of CFT measurement were analyzed in patients with available OCT findings at both baseline and 12 months posttreatment. Subgroup analyses of the parameters were performed as described below.
The patients were divided into groups based on the difference in baseline BCVA between the two eyes. In patients with different baseline BCVA in the two eyes, the eye that exhibited the worse visual acuity was included in the worse visual acuity group, and the fellow eye was included in the better visual acuity group. The baseline and 12-month BCVA values were compared within each group. In addition, the 12-month changes in BCVA were compared between the two groups. In patients that exhibited equivalent baseline BCVA in both eyes (<0.1 logarithm of minimal angle of resolution [logMAR]), the proportions of the pairs of eyes that exhibited equivalent BCVA, 1 to 2 lines of difference in BCVA, and ≥3 lines of difference in BCVA at 12 months were estimated.
The patients were divided into groups based on baseline RAP stage in both eyes. In patients with different stages of RAP in the two eyes, eyes with stage 1 RAP were included in the lower stage group, and the fellow eyes (stage 2 RAP) were included in the higher stage group. The baseline and 12-month BCVA values were compared within each group. In addition, the 12-month changes in BCVA were compared between the two groups. In patients that exhibited the same stage of RAP in both eyes, the proportions of pairs of eyes that exhibited equivalent BCVA values, 1 to 2 lines of difference in BCVA, and ≥3 lines of difference in BCVA at baseline and 12 months posttreatment were estimated.
The eyes were classified into two groups according to timing of RAP symptom onset. The 12-month BCVA values were compared between the eyes with early and late onset of symptoms. Additionally, the baseline and 12-month BCVA values were compared within each group.
For all 38 eyes included in the study, the presence of intra and subretinal fluids and pigment epithelial detachment (PED) at baseline was identified based on OCT findings. The 12-month BCVA values were compared between eyes with and without these pathological findings. In addition, the baseline and 12-month BCVA values were compared within each group.
Statistical analyses were performed using the commercially available software package SPSS ver. 12.0 for Windows (SPSS Inc., Chicago, IL, USA). The number of anti-VEGF injections between the subgroups was compared using the independent samples t-test or Mann-Whitney U-test. Comparison of the BCVA values at different time points was performed using the paired t-test or Wilcoxon signed-rank test. The paired t-test was used to compare the CFT at different time points, and comparison of the BCVA values between different subgroups was performed using the independent samples t-test or Mann-Whitney U-test. The p-values of <0.05 were considered statistically significant.
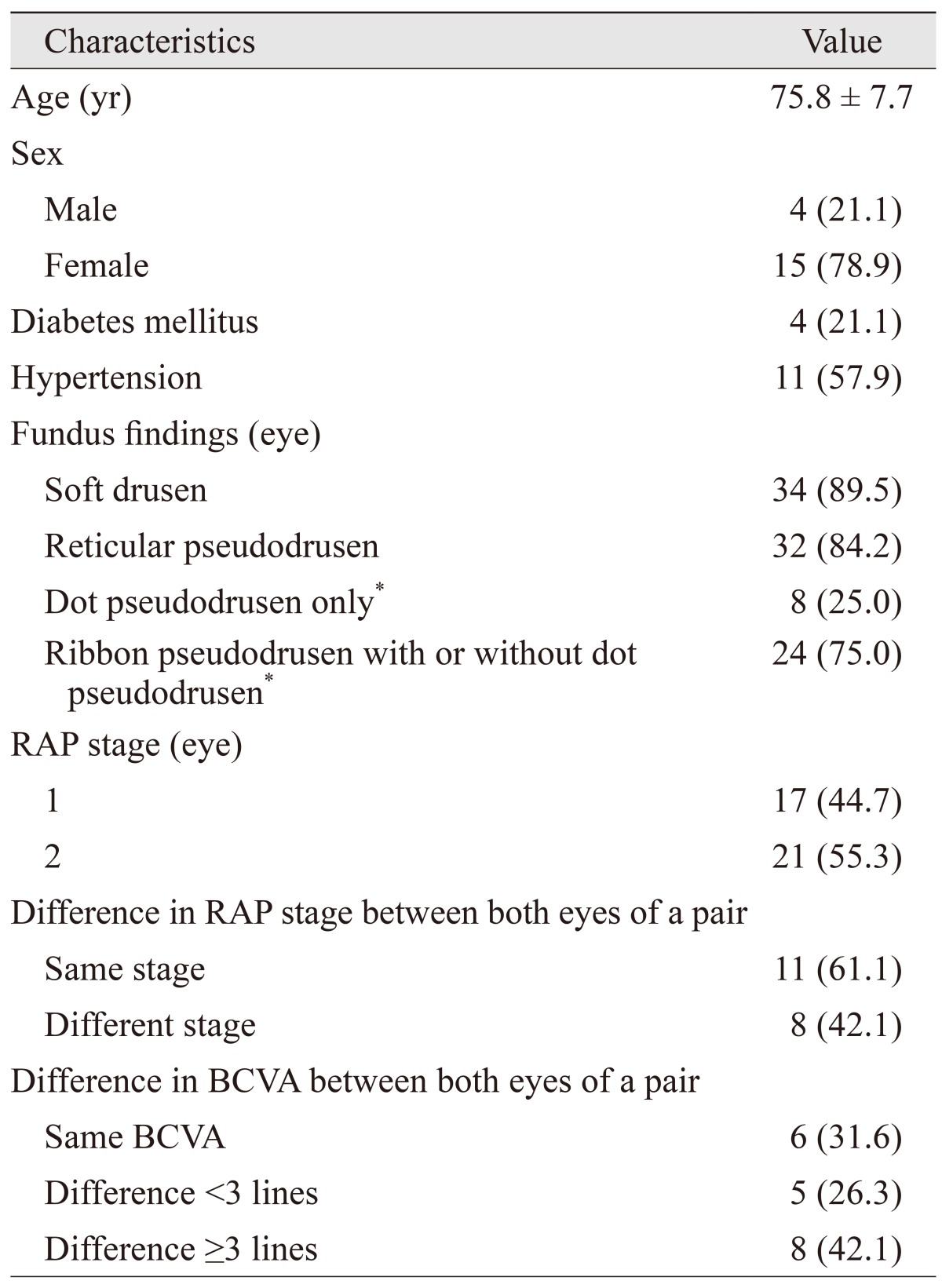
The baseline characteristics of the 19 patients (38 eyes) who satisfied the eligibility criteria are summarized in Table 1. Of the 19 patients, four (21.1%) were male and the mean patient age was 75.8 ± 7.7 years. Diabetes mellitus and hypertension were present in four (21.1%) and 11 (57.9%) patients, respectively. Stage 1 and 2 RAP lesions were observed in 17 (44.7%) and 21 (55.3%) eyes, respectively. While six patients (31.6%) exhibited equivalent BCVA in both eyes, five (26.3%) exhibited 1 to 2 lines of difference in BCVA between the two eyes, and the remaining eight patients (42.1%) exhibited ≥3 lines of difference in BCVA between the two eyes.
During the 12-month follow-up period, the patients received an average of 4.3 ± 0.8 anti-VEGF injections (ranibizumab injections, 3.9 ± 0.8; bevacizumab injections, 0.3 ± 0.6) in each eye. Nine eyes were treated with both ranibizumab and bevacizumab, and the remaining 29 were treated with ranibizumab alone. Images of a representative case of bilateral RAP treated with an anti-VEGF agent are shown in Fig. 1A-1J. The mean BCVA values of the 38 eyes at baseline and 3 and 12 months posttreatment were 0.63 ± 0.26, 0.49 ± 0.29, and 0.71 ± 0.34, respectively. The BCVA at 3 months posttreatment was significantly better than the BCVA at baseline (p=0.001). However, there was a significant decrease in mean BCVA between 3 and 12 months posttreatment (p<0.001). Therefore, the BCVA at 12 months posttreatment was not significantly different from that at baseline (p=0.192).
A significant association was found in mean 12-month change in BCVA between the eyes of a pair (p<0.001, r=0.739) (Fig. 2). Thus, the eye that exhibited better visual acuity than the paired eye at baseline was likely to continue to exhibit better visual acuity than the other eye at 12 months posttreatment. The CFT measurements were analyzed in 34 eyes of 17 patients (87.2%) with available OCT results at baseline and at 3 and 12 months posttreatment. The CFT values at baseline and 3 and 12 months posttreatment were 372.5 ± 111.4, 225.4 ± 67.6, and 266.7 ± 136.4 µm, respectively. The mean CFT values at 3 and 12 months posttreatment were significantly lower than those at baseline (p<0.001, both). There was no significant difference between the mean CFT values at 3 and 12 months posttreatment (p=0.150).
Of the 19 pairs of eyes included in the study, 13 exhibited different baseline BCVA values in paired eyes (Fig. 3A). In the better visual acuity group (n=13), the BCVA values at baseline and 3 and 12 months posttreatment were 0.48 ± 0.19, 0.35 ± 0.19, and 0.58 ± 0.29, respectively, and the BCVA at baseline was not significantly different from that at 12 months posttreatment (p=0.174). These eyes were treated with an average of 4.5 ± 0.8 anti-VEGF injections during the 12-month period. In the worse visual acuity group (n=13), the BCVA values at baseline and 3 and 12 months posttreatment were 0.83 ± 0.20, 0.64 ± 0.30, and 0.90 ± 0.31, respectively, and the BCVA at baseline was not significantly different from that at 12 months posttreatment (p=0.460). There was no significant difference in 12-month change in BCVA (p=0.786) or number of anti-VEGF injections (p=0.111) between the eyes with better and worse visual acuity.
Of the six patients who exhibited equivalent baseline BCVA in both eyes, five exhibited the same stage of RAP in both eyes. Equivalent BCVA values in paired eyes were noted in two patients (33.3%) at 12 months posttreatment. The remaining four patients exhibited 1 to 2 lines or ≥3 lines of difference in BCVA (two patients [33.3%] each) at 12 months posttreatment (Fig. 3B).
Of the total 19 patients, eight exhibited different stages of RAP in paired eyes at baseline (Fig. 4A). In the lower stage group (n=8), the BCVA values at baseline and 3 and 12 months posttreatment were 0.48 ± 0.17, 0.36 ± 0.23, and 0.54 ± 0.35, respectively, and the BCVA at baseline was not significantly different from that at 12 months posttreatment (p=0.892). In the higher stage group (n=8), the BCVA values at baseline and 3 and 12 months posttreatment were 0.78 ± 0.27, 0.72 ± 0.35, and 0.89 ± 0.36, respectively, and the BCVA at baseline was not significantly different from that at 12 months posttreatment (p=0.408). There were no significant differences in the 12-month change in BCVA (p=0.505) and the number of anti-VEGF injections (p=0.279) between the eyes of the lower and the higher stage groups.
Of the 11 patients that exhibited the same RAP stages in paired eyes at baseline, five (45.5%) exhibited equivalent baseline BCVA in both eyes, two (18.2%) exhibited 1 to 2 lines of difference in the BCVA of the two eyes at baseline, and the remaining four patients (36.4%) exhibited ≥3 lines of difference in the BCVA of the two eyes. At 12 months posttreatment, equivalent BCVA in both eyes and 1 to 2 and ≥3 lines of difference in the BCVA of paired eyes were observed in two (18.2%), four (36.4%), and five (45.5%) patients, respectively (Fig. 4B).
Among the 19 patients included in the study, the timing of onset of symptoms was not accurately determined in four. Of the 15 patients with records of the timing of symptom onset, six had similar timing of onset for the two eyes. Therefore, comparison between the early and late onset eyes was performed for the nine patients (18 eyes) that had dissimilar timing of symptom onset. The BCVA values at baseline and 12 months posttreatment in the early onset eyes (n=9) were 0.72 ± 0.33 and 0.76 ± 0.43, while those in the late onset eyes (n=9) were 0.49 ± 0.23 and 0.50 ± 0.26, respectively. Compared to the BCVA at baseline, that at 12 months posttreatment was significantly lower in the early onset eyes (p=0.031), whereas the difference between the two was not significant in the late onset eyes (p=0.094). Although the early onset eyes showed better visual acuity at 12 months posttreatment than the late onset eyes, there was no significant difference in BCVA between the two groups at 12 months posttreatment (p=0.258).
The incidences of intra and subretinal fluid accumulation and PED were 100% (38 eyes), 21.1% (eight eyes), and 71.1% (27 eyes), respectively. The BCVA values at baseline and 12 months posttreatment in eyes with subretinal fluid accumulation were 0.63 ± 0.27 and 0.67 ± 0.41, while those in eyes without subretinal fluid accumulation were 0.63 ± 0.27 and 0.71 ± 0.32, respectively. There was no significant difference in the 12-month BCVA between the two groups (p=0.750). The BCVA at baseline was not significantly different from that at 12 months posttreatment in eyes with or without subretinal fluid accumulation (p=0.188 and p=0.063, respectively).
The BCVA values at baseline and 12 months posttreatment in eyes with PED were 0.69 ± 0.26 and 0.79 ± 0.35, while those in eyes without PED were 0.47 ± 0.19 and 0.51 ± 0.23, respectively. The BCVA at 12 months posttreatment was significantly better in eyes without PED (p=0.021) than in those with PED. The BCVA at baseline was not significantly different from that at 12 months posttreatment in eyes with or without PED (p=0.085 and p=0.527, respectively).
In this study, the overall treatment outcomes were similar or slightly unfavorable compared to those reported in previous studies [12,14,15]. The 12-month visual acuities of our patients showed deterioration compared with the corresponding baseline values, although the differences between the two were not statistically significant. Previous studies that evaluated the long-term visual outcome of anti-VEGF therapy in RAP reported 12-month visual acuities significantly better than [12] or similar to [14] the baseline values. Although a previous study reported 12-month visual outcomes that were similar to those identified in our study [15], the anti-VEGF monotherapy group in that study included patients with relatively high-grade RAP (stage 2, 76.9%; stage 3, 23.1%). The relatively discouraging visual outcomes of our patients, in spite of excluding stage 3 RAP, which is usually considered to have poor prognosis [17], requires further research. In this study, the BCVA at 3 months posttreatment, which was measured 1 month after the initial monthly anti-VEGF injections, significantly improved from that at baseline. However, there was also a significant deterioration in BCVA between 3 and 12 months posttreatment. As a result, the BCVA at 12 months posttreatment was not significantly different from that at baseline. This result suggests that at least some of our cases were under-treated. In a previous prospective study that followed a frequent follow-up schedule [12], an average of 5.5 anti-VEGF injections were administered during the first 12 months of treatment; in comparison, the average number of anti-VEGF injections administered in the present study was 4.3. In addition, in the present study, follow-up examinations were performed every 1 to 3 months at the discretion of the treating physician. We hypothesize that the relatively small number of injections and poor visual prognosis in our patients are mainly attributable to the infrequent follow-up strategy, which might have caused delayed treatment and under-treatment.
Although the prevalence of reticular pseudodrusen is relatively high among patients with RAP [20,21,22], the prevalence among the patients included in our study (84.2%) was higher than that reported among patients in other studies on RAP. The high prevalence observed among our patients may be partially attributable to the inclusion criteria of the present study (i.e., presence of bilateral RAP) because the presence of reticular pseudodrusen in the fellow eye is closely associated with fellow-eye neovascularization in patients with unilateral RAP [4,5]. The presence of reticular pseudodrusen is also associated with choroidal thinning [23,24]. This was confirmed by a recent study that demonstrated poor treatment outcomes in patients with exudative AMD with thin choroid [25]. Therefore, we hypothesized that the high incidence of reticular pseudodrusen contributed to the relatively poor treatment outcomes in our patients.
In general, regardless of baseline visual acuity or stage of RAP, there were no notable differences in the response to therapy between both eyes of a pair. However, despite comparable baseline visual acuities, the treatment outcomes were not always similar between the paired eyes of the patients. Equivalent BCVA values at 12 months posttreatment were observed in only two of the six patients with equivalent baseline BCVA values, although five of the six patients exhibited the same stage of RAP in both eyes. The 12-month visual acuities of the eyes with worse baseline visual acuities were generally inferior compared to those of the fellow eyes with better baseline values. This result was in accordance with a previous study that demonstrated a close positive association between baseline and posttreatment visual acuities [14]. There were no significant differences in 12-month changes in visual acuities between the eyes of the worse and better visual acuity groups. In a previous study that evaluated the short-term outcomes of anti-VEGF therapy in bilateral exudative AMD, the increase in BCVA after three anti-VEGF injections was significantly greater in eyes with worse baseline visual acuities compared to that in eyes with better baseline values [26]. However, no such trend was observed in the 12-month changes in visual acuities among our patients.
In the present study, the visual acuity at 12 months posttreatment was relatively better in the late onset eyes than in the early onset eyes. Although the mean difference in visual acuity between the two groups was more than 0.2 logMAR lines, it was not a statistically significant difference. We postulate that this result might have primarily been due to the small sample size of this study. The relatively better visual outcome in the late onset eyes compared to that in the early onset eyes suggests that better visual outcome can be achieved by initiating treatment at earlier stages of the disease.
Previous studies on the OCT features of RAP have reported relatively high incidences of intraretinal fluid and PED and low incidence of subretinal fluid [27,28,29]. In addition, the choroid has been reported to be usually thin in RAP [30]. In the present study, the BCVA outcomes were compared between eyes with or without subretinal fluid or PED. The other characteristic OCT features of RAP were not analyzed for the following reasons: intraretinal fluid because all of the patients included in the present study exhibited accumulation of intraretinal fluid; choroidal thickness because enhanced-depth imaging OCT, which is required for accurate choroidal thickness measurement, was not performed in some of the patients included in our study. The results of our study indicate better 12-month visual outcome in eyes without PED than in eyes with PED. It has been reported that PED is associated with poor visual outcome in neovascular AMD [31]. Our results suggest that the presence of PED in RAP is also a predictive clinical factor of worse visual outcome.
This study was the first to evaluate detailed outcomes of bilateral RAP. However, this study also had some limitations, including the retrospective nature and small sample size. Although initial treatment was performed according to uniform guidelines, no such guidelines were followed during re-treatment, which was performed according to the discretion of each clinician. Finally, the monthly follow-up schedule, which is generally recommended in treatment with as-needed regimens [32], was not strictly followed.
In summary, the response to treatment with anti-VEGF was comparable between the eyes of the better and worse visual acuity groups in patients with bilateral RAP. The treatment outcomes might vary markedly in some cases with similar baseline visual acuities. Better baseline visual acuity and absence of PED at diagnosis were associated with better visual acuity at 12 months posttreatment. However, the stage of RAP at baseline was not associated with the treatment outcome. Therefore, additional controlled studies with strict treatment protocols and follow-up schedules are required to better elucidate the treatment outcomes in bilateral RAP.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
REFERENCES
1. Yannuzzi LA, Negrao S, Iida T, et al. Retinal angiomatous proliferation in age-related macular degeneration. Retina 2001;21:416-434.


2. Gross NE, Aizman A, Brucker A, et al. Nature and risk of neovascularization in the fellow eye of patients with unilateral retinal angiomatous proliferation. Retina 2005;25:713-718.


3. Campa C, Harding SP, Pearce IA, et al. Incidence of neovascularization in the fellow eye of patients with unilateral retinal angiomatous proliferation. Eye (Lond) 2010;24:1585-1589.


4. Sawa M, Ueno C, Gomi F, Nishida K. Incidence and characteristics of neovascularization in fellow eyes of Japanese patients with unilateral retinal angiomatous proliferation. Retina 2014;34:761-767.


5. Chang YS, Kim JH, Yoo SJ, et al. Fellow-eye neovascularization in unilateral retinal angiomatous proliferation in a Korean population. Acta Ophthalmol 2016;94:e49-e53.


6. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419-1431.


7. CATT Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011;364:1897-1908.



8. Kang S, Roh YJ. One-year results of intravitreal ranibizumab for neovascular age-related macular degeneration and clinical responses of various subgroups. Jpn J Ophthalmol 2009;53:389-395.


9. Chang YS, Han JI, Yoo SJ, et al. Intravitreal anti-vascular endothelial growth factor for typical exudative age-related macular degeneration in eyes with good baseline visual acuity. Korean J Ophthalmol 2014;28:466-472.



10. Lee SY, Kim JG, Joe SG, et al. The therapeutic effects of bevacizumab in patients with polypoidal choroidal vasculopathy. Korean J Ophthalmol 2008;22:92-99.



11. Hemeida TS, Keane PA, Dustin L, et al. Long-term visual and anatomical outcomes following anti-VEGF monotherapy for retinal angiomatous proliferation. Br J Ophthalmol 2010;94:701-705.


12. Gharbiya M, Parisi F, Cruciani F, et al. Intravitreal anti-vascular endothelial growth factor for retinal angiomatous proliferation in treatment-naive eyes: long-term functional and anatomical results using a modified PrONTO-style regimen. Retina 2014;34:298-305.


13. Parodi MB, Iacono P, Menchini F, et al. Intravitreal bevacizumab versus ranibizumab for the treatment of retinal angiomatous proliferation. Acta Ophthalmol 2013;91:267-273.


14. Inoue M, Arakawa A, Yamane S, Kadonosono K. Long-term results of intravitreal ranibizumab for the treatment of retinal angiomatous proliferation and utility of an advanced RPE analysis performed using spectral-domain optical coherence tomography. Br J Ophthalmol 2014;98:956-960.


15. Rouvas AA, Chatziralli IP, Theodossiadis PG, et al. Long-term results of intravitreal ranibizumab, intravitreal ranibizumab with photodynamic therapy, and intravitreal triamcinolone with photodynamic therapy for the treatment of retinal angiomatous proliferation. Retina 2012;32:1181-1189.


16. Lai TY, Chan WM, Liu DT, Lam DS. Ranibizumab for retinal angiomatous proliferation in neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2007;245:1877-1880.


17. Reche-Frutos J, Calvo-Gonzalez C, Perez-Trigo S, et al. Ranibizumab in retinal angiomatous proliferation (RAP): influence of RAP stage on visual outcome. Eur J Ophthalmol 2011;21:783-788.


18. Konstantinidis L, Mameletzi E, Mantel I, et al. Intravitreal ranibizumab (Lucentis) in the treatment of retinal angiomatous proliferation (RAP). Graefes Arch Clin Exp Ophthalmol 2009;247:1165-1171.


19. Kang JH, Park KA, Chung SE, Kang SW. Retinal angiomatous proliferation and intravitreal bevacizumab injection. Korean J Ophthalmol 2007;21:213-215.



20. Cohen SY, Dubois L, Tadayoni R, et al. Prevalence of reticular pseudodrusen in age-related macular degeneration with newly diagnosed choroidal neovascularisation. Br J Ophthalmol 2007;91:354-359.


21. Ueda-Arakawa N, Ooto S, Nakata I, et al. Prevalence and genomic association of reticular pseudodrusen in age-related macular degeneration. Am J Ophthalmol 2013;155:260-269.e2.


22. Kim JH, Chang YS, Kim JW, et al. Prevalence of subtypes of reticular pseudodrusen in newly diagnosed exudative age-related macular degeneration and polypoidal choroidal vasculopathy in Korean patients. Retina 2015;35:2604-2612.


23. Haas P, Esmaeelpour M, Ansari-Shahrezaei S, et al. Choroidal thickness in patients with reticular pseudodrusen using 3D 1060-nm OCT maps. Invest Ophthalmol Vis Sci 2014;55:2674-2681.



24. Ueda-Arakawa N, Ooto S, Ellabban AA, et al. Macular choroidal thickness and volume of eyes with reticular pseudodrusen using swept-source optical coherence tomography. Am J Ophthalmol 2014;157:994-1004.


25. Kang HM, Kwon HJ, Yi JH, et al. Subfoveal choroidal thickness as a potential predictor of visual outcome and treatment response after intravitreal ranibizumab injections for typical exudative age-related macular degeneration. Am J Ophthalmol 2014;157:1013-1021.


26. Jonas JB, Tao Y, Rensch F. Bilateral intravitreal bevacizumab injection for exudative age-related macular degeneration: effect of baseline visual acuity. J Ocul Pharmacol Ther 2011;27:401-405.


27. Matsumoto H, Sato T, Kishi S. Tomographic features of intraretinal neovascularization in retinal angiomatous proliferation. Retina 2010;30:425-430.


28. Rouvas AA, Papakostas TD, Ntouraki A, et al. Angiographic and OCT features of retinal angiomatous proliferation. Eye (Lond) 2010;24:1633-1642.


29. Kim JH, Chang YS, Kim JW, et al. Diagnosis of type 3 neovascularization based on optical coherence tomography images. Retina 2016;36:1506-1515.


30. Kim JH, Kim JR, Kang SW, et al. Thinner choroid and greater drusen extent in retinal angiomatous proliferation than in typical exudative age-related macular degeneration. Am J Ophthalmol 2013;155:743-749. 749.e1-749.e2.


Fig. 1
A representative case showing treatment outcome of bilateral retinal angiomatous proliferation. At diagnosis (A-F), the visual acuities of the right and left eyes were 20 / 50 and 20 / 50, respectively (arrows in panels C and D indicate the retinal angiomatous proliferation lesion). The eyes were treated with anti-vascular endothelial growth factor for 12 months. At 12 months posttreatment (G-J), despite a mild recurrence of exudation in the left eye, the visual acuities of the right (G,I) and left (H,J) eyes had improved to 20 / 25 and 20 / 30, respectively. (A,B,G,H): Fundus photography images; (C,D): indocyanine green angiography images; (E,F,I,J): optical coherence tomography images.

Fig. 2
Scatterplot showing the association of baseline and 12-month logarithm of minimal angle of resolution (logMAR) best-corrected visual acuity (BCVA) values between the eyes of a pair. Positive values indicate better visual acuity of the right eye compared to that of the left eye, and negative values indicate better visual acuity of the left eye compared to that of the right eye.

Fig. 3
Results of best-corrected visual acuity (BCVA) evaluation in eyes with bilateral retinal angiomatous proliferation divided into subgroups according to the baseline VA. (A) Changes in BCVA in 13 patients exhibiting different BCVA values in both eyes at baseline. Closed circles (solid line) indicate eyes with better baseline VA, and closed squares (dashed line) indicate eyes with worse baseline VA. (B) Changes in the proportions of patients exhibiting the equivalent VA, 1 to 2 lines of difference in BCVA, and ≥3 lines of difference in BCVA in both eyes from baseline to 12 months posttreatment. These data were derived from six patients with equivalent baseline BCVA in both eyes. logMAR = logarithm of minimal angle of resolution.

Fig. 4
Results of best-corrected visual acuity (BCVA) evaluation in eyes with bilateral retinal angiomatous proliferation (RAP) divided into subgroups according to stage of disease. (A) Changes in BCVA in eight patients exhibiting different stages of RAP in paired eyes. Closed circles (solid line) indicate eyes with lower stage (stage 1) of disease, and closed squares (dashed line) indicate eyes with higher stage (stage 2) of disease. (B) Changes in the proportions of patients exhibiting equivalent visual acuity, 1 to 2 lines of difference in BCVA, and ≥3 lines of difference in BCVA in paired eyes from baseline to 12 months posttreatment. These data were derived from 11 patients with the same stage of RAP in paired eyes. logMAR = logarithm of minimal angle of resolution.

- TOOLS
-
METRICS

-
- 4 Crossref
- 0 Scopus
- 2,379 View
- 23 Download
- Related articles



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



