Choroidal Thickness Variation According to Refractive Error Measured by Spectral Domain-optical Coherence Tomography in Korean Children
Article information
Abstract
Purpose
To assess choroidal thickness (CT) variation according to refractive errors using enhanced-depth imaging optical coherence tomography.
Methods
Eighty-nine eyes (in 89 children) <±6 diopter were categorized into three groups: hyperopia, emmetropia, and myopia, according to refractive error, and underwent choroidal scans using enhanced-depth imaging-optical coherence tomography. CT was measured at the fovea and at 1 mm and 3 mm nasal (N1 and N3), temporal (T1 and T3), superior (S1 and S3), and inferior (I1 and I3) from the fovea.
Results
Mean foveal CTs were 346.86 µm, 301.97 µm, and 267.46 µm in the hyperopia, emmetropia, and myopia groups, respectively (p < 0.05). CTs at N3 and T3 were 214.59 µm and 318.68 µm, 163.92 µm and 320.79 µm, and 153.93 µm and 295.61 µm in the hyperopia, emmetropia, and myopia groups, respectively (p < 0.05). All CTs in the hyperopia group were thicker than those of other groups (p < 0.05). Fovea was thickest and was significantly thicker than at N3 and I3 in hyperopia (p < 0.05). T3 thickness in the emmetropia and myopia groups was greater than thickness at other areas, particularly the nasal and inferior choroids (p < 0.05). CT was positively correlated with spherical equivalent (p = 0.029).
Conclusions
In Korean children, CTs were greater in the hyperopia group than in the emmetropia and myopia groups. The temporal choroid was thicker than the nasal choroid, regardless of the refractive error. The thickest location in the hyperopia group was the fovea; however, the temporal choroid was thickest in the emmetropia and myopia groups.
Introduction
The choroid plays a crucial role in maintaining retinal and visual functions, such as providing nutrients to the external retina and the retinal pigment epithelial cells and supplying blood to the anterior layer of the optic nerves [1].
The development of optical coherence tomography (OCT) has enabled direct observation of the choroid in vivo, and allows quantitative observations, such as measuring its thickness. As a result, OCT has been used in many studies [23456].
The choroidal thickness is known to vary with factors including ethnicity, age, and time of day and with ocular factors including axial length and refractive errors [789]. Various studies have reported that greater axial length and myopia are associated with a thinner choroid [10]. The first study of choroidal thickness profiles of healthy Korean children was conducted in 2013 [11]. In this study, mean subfoveal choroidal thickness in children was greater compared to choroidal thicknesses from previous studies in healthy adults. In addition, subfoveal choroidal thickness showed a significant negative correlation with age. However, few studies have investigated the distribution of the choroid thickness according to refractive error in children of a similar age, and no such study has been conducted in Korean children.
Therefore, in this study, we compared the distribution of choroid thickness in children with refractive errors, such as hyperopia or myopia, with that in children without refractive errors, using enhanced-depth imaging-OCT.
Materials and Methods
Materials
This is a cross-sectional comparative, non-interventional study conducted at Cheil Eye Hospital in Daegu, Republic of Korea, from June to September in 2011. The protocol of this study conformed to the tenets of the Declaration of Helsinki and was approved by the institutional review board of Cheil Eye Hospital.
Eighty-nine children (89 eyes), aged between 6 and 12 years, participated in this study. All subjects completed a visual acuity assessment and underwent spectral domain-OCT to evaluate the retina and choroid. All enrolled subjects had best-corrected visual acuity in both eyes of 0.00 logarithm of the minimal angle of resolution or better, refractive errors with less than ±6 diopter, and no evidence or history of contact lens use, significant ocular disease, eye surgery or injury. All subjects were in good general health and without congenital, neurological, or other disorder. In addition, subjects underwent a slit lamp examination, cycloplegic refraction, best-corrected visual acuity assessment, fundoscopy, and measurement of axial length and posterior choroidal thickness.
For cycloplegic refraction, 1% cyclopentolate hydrochlo-ride (Cyclogyl; Alcon, Fort Worth, TX, USA) was administered dropwise, twice or more, into each eye at 5-minute intervals; 30 to 40 minutes after its administration, refractive errors were measured when pupil dilation and loss of light reflex were confirmed.
Subjects were classified into three groups based on refractive error: those with a +1 diopter or greater refractive power were assigned to the hyperopia group; those with a diopter lower than +1 and greater than −1 were assigned to the emmetropia group; and those with a −1 diopter or lower diopter were assigned to the myopia group.
Method
Analysis of choroid was completed using the enhanced-depth imaging method of spectral domain-OCT (Spectralis OCT; Heidelberg Engineering, Heidelberg, Germany) before cycloplegic refraction. Two experienced examiners (JHL, GYL) measured the choroidal thickness— the perpendicular distance from the external border of the retinal epithelial pigment with a high reflective layer to the internal scleral interface with a high reflective layer—using a built-in caliper. The measurement was performed after vertical and horizontal choroid images were taken with default K (7.7 mm) and signal strength over 8. The nasal points 1 mm and 3 mm from the fovea were set as N1 and N3; in addition, the temporal points 1 mm and 3 mm from the fovea were set as T1 and T3. The points 1 mm and 3 mm superior from the fovea were set as S1 and S3, and the points 1 mm and 3 mm inferior to the fovea were set as I1 and I3. The axial length was measured using a partial coherence interferometer IOL Master (Carl Zeiss Meditec, Dublin, CA, USA). The choroid was measurable in all cases, and the signal-to-noise ratio was 2.1 or higher.
Statistical analysis was performed using PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA). To determine the difference between the choroidal thickness values of the points on the retina, the mean and standard deviation of age, spherical equivalent, and axial length were compared using one-way analysis of variance. Comparisons of choroidal thickness at different measurement points were analyzed according to the refractive error, with post-hoc comparisons by Fisher's least significant difference (LSD) adjustment. A multiple linear regression model with stepwise selection was used to evaluate correlations between choroidal thickness and refractive error, age, and axial length. To calculate interobserver variability, intraclass correlation coefficient was analyzed. The p-values <0.05 were considered significant.
Results
A total of 89 eyes (in 89 children) were investigated: 40 subjects were boys and 49 were girls. The mean age, diopter, and axial length are shown in Table 1. The choroid was thickest at T3 and thinnest at N3 (Table 2 and Fig. 1A). The foveal measurement was thicker than the superior or inferior results; however, I3 was the thinnest area in the vertical cross-section (p < 0.05; post-hoc LSD test) (Fig. 1B).

The choroidal thicknesses at various locations with respect to the fovea according to refractive errors
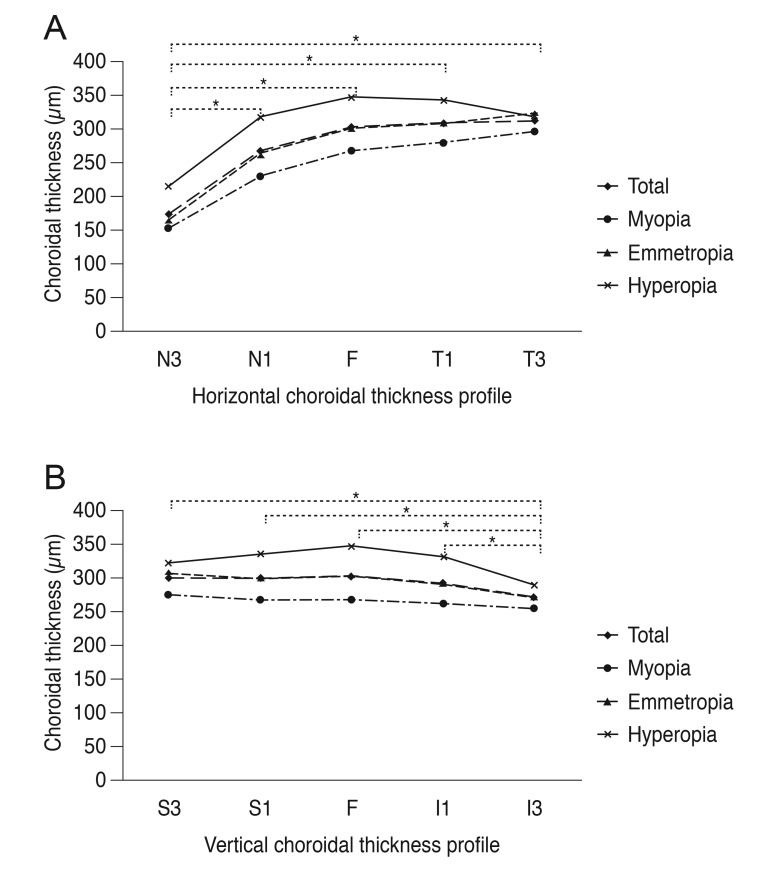
Horizontal (A) and vertical (B) choroidal thickness profiles. (A) N3–N1, p < 0.001; N3–F, p < 0.001; N3–T1, p < 0.001; N3–T3, p < 0.001. (B) S3–I3, p = 0.001; S1–I3, p = 0.003; F–I3, p = 0.001; I1–I3, p = 0.014. N3 = 3 mm nasal to fovea; N1 = 1 mm nasal to fovea; F = fovea; T1 = 1 mm temporal to fovea; T3 = 3 mm temporal to fovea; S3 = 3 mm superior to fovea; S1 = 1 mm superior to fovea; I1 = 1 mm inferior to fovea; I3 = 3 mm inferior to the fovea. *p-value <0.05 using post-hoc analysis with least significant difference test in all eyes.
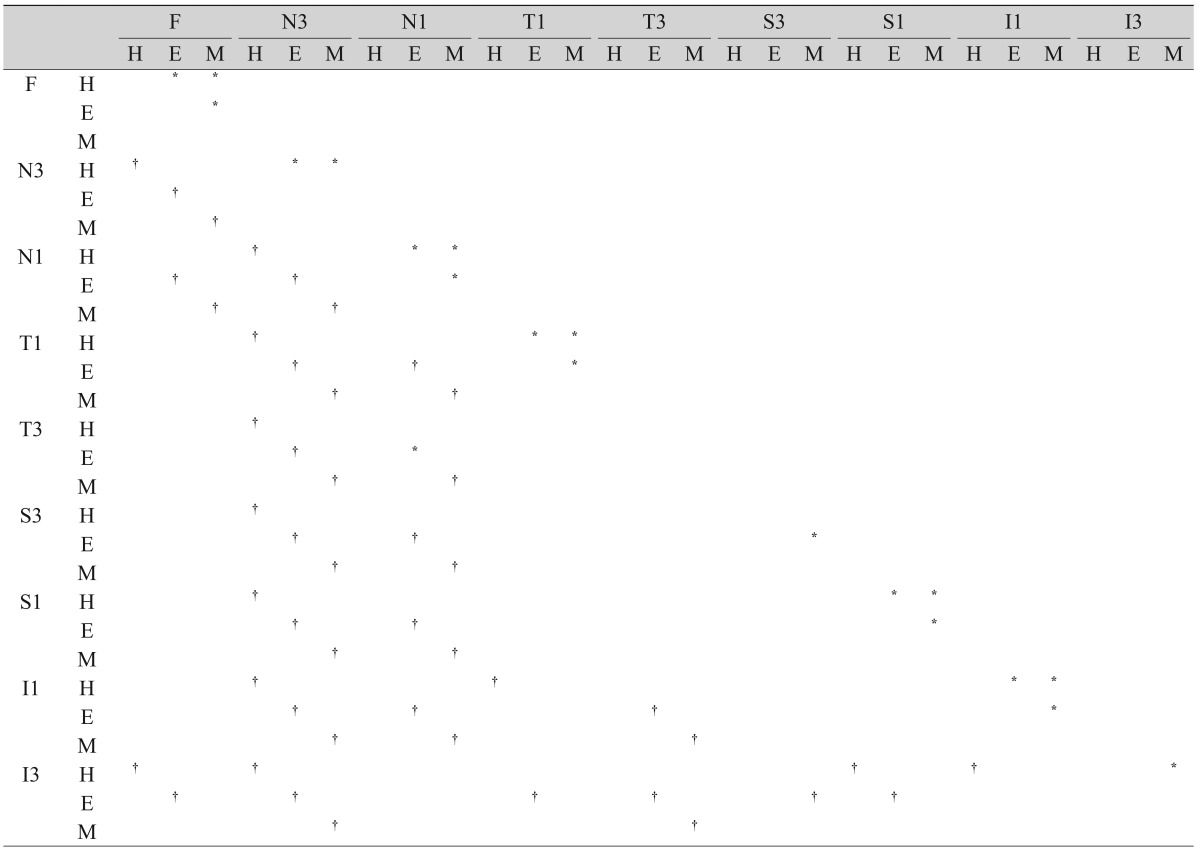
The choroidal thicknesses, as measured at various points on the retina, are given in Table 2. The choroidal thickness was the thinnest at N3, regardless of refractive errors (p < 0.05, post-hoc LSD test) (Table 3 and Fig. 1A). In the hyperopia group, the choroidal thicknesses at S1, I1, T1, and F were thicker than at I3 (p = 0.023, p = 0.031, p = 0.009, p = 0.005, respectively; post-hoc LSD test) (Table 3). The superior, temporal, and foveal choroids were thicker than that in the nasal area in the emmetropia and myopia groups (p < 0.005, post-hoc LSD test) (Table 3). In the emmetropia group, choroidal thicknesses at S3, S1, and the foveal choroid were thicker than that at I3 in the vertical section across the fovea (p = 0.005, p = 0.026, p = 0.016; post-hoc LSD test); however, there was no statistical difference between superior and inferior choroidal thickness in myopia (Table 3).
Choroidal thickness at different measurement points also varied according to the refractive error present. Choroidal thicknesses at N3, N1, T1, S1, I1, and the fovea in the hyperopia group were significantly greater than in the emmetropia group (Table 3). Choroidal thicknesses at S3, S1, I1, N1, T1, and the fovea in the emmetropia group were significantly thicker than in the myopia group (Table 3). All the points, with the exception of T3, were significantly thicker in the hyperopia group than in the myopia group (Table 3).
Choroidal thickness at each retinal point was positively correlated with spherical equivalent (p = 0.029, r2 = 0.306). However, the correlations between choroidal thickness with axial length and age were not significant (p = 0.265 and p = 0.149, respectively).
The reliability of choroidal thickness measurements between two examiners was assessed by intraclass correlation coefficient which was >0.990 (p < 0.01).
Discussion
In this study, we compared choroidal thickness based on refractive errors in children of similar ages for the first time. We found that choroidal thickness at the fovea is thicker in children with hyperopia (346.86 µm) than in those with emmetropia (301.97 µm) or myopia (267.46 µm); thus, choroidal thickness differs according to the refractive error present.
According to results from some animal studies, changes in choroidal thickness may be due to the maintenance of clear vision. Previous studies in chicks, marmosets, and macaques have led to the hypothesis that induced myopic defocus leads to choroidal thickening due to adjustment of the retina location and to maintain clear vision. This could result because in myopic defocus the image plane is in front of the retina so that a thickening of the choroid pushes the retina forward the image plane. Data from previous studies have shown that choroidal thickening, as a part of this compensation, inhibits the penetration of various growth factors that function as mechanical barriers and slows the growth of sclera [121314]. Thus, results from these studies are consistent with data from this study showing that refractive error leads to choroidal thickening in hyperopia in order to maintain clear vision, and to choroidal thinning in myopia.
In a study on hyperopic anisometropic amblyopia, the choroidal thickness of the fovea in the affected eye (351.3 µm) was greater than that in the healthy eye (283.5 µm). This study, conducted in 25 patients, reported a mean refractive error of +3.97 diopter and a mean age of 6.6 years, which are similar to the results of this study [15]. In another study on nanophthalmos, or severe hyperopia with a mean refractive error of +10.6 diopter and an axial length of 18.8 mm, the choroidal thickness at the fovea was 551.3 µm, which was greater than that observed in this study [16]. This difference could be due to subjects of the earlier study having greater hyperopic refractive errors and a shorter axial length than those in this study, even though changes in the scleral collagen fibers and glycosaminoglycan metabolism, and the production of fibronectin by sclera cells, differed between the normal eyes and the nanophthalmic eyes [17]. In this study, we found that the only factor influencing choroidal thickness was the refractive error using multiple regression model. Similarly, in a recent study on anisometropic hyperopic amblyopia, hyperopic nonamblyopic and emmetropic eyes, there were no significant differences in subfoveal choroidal thickness between hyperopic nonamblyopic and hyperopic anisometropic amblyopic eyes [18]. Furthermore, in this study, amblyopia had no independent effect on choroidal thickness in hyperopic eyes, although spherical equivalent was significantly associated with choroidal thickness.
Previous studies on myopia have shown that adult myopia patients have thinner choroids [10]. In this study, the choroidal thickness in myopic eyes was 263.94 µm and was thinner than the choroids in nonmyopic eyes. A previous study reported a mean foveal choroidal thickness of 303 µm in patients with myopia compared to 359 µm in those with emmetropia, which was consistent with our findings [4].
In terms of choroidal thickness at each measurement point, the thickest point was at the fovea in the hyperopia group, and at T3 in the emmetropia group. The superior and temporal choroid was thicker than the inferior and nasal choroid in both groups. In the emmetropia group, the thickness of the choroid at the fovea was thinner than at the temporal position from the fovea, but was thicker than that at the nasal points from the fovea. These results were consistent with data from a study on hyperopic anisometropic amblyopia that reported greatest choroid thickness at the fovea in eyes with hyperopia and amblyopia, and at the point temporal to the macula in the healthy eye [15]. However, our results indicate that the thicker choroid in the fovea of the affected eye was attributable to hyperopia rather than amblyopia.
Some studies have reported that choroidal thickness differs depending on the position of the measurement points in children. In a study by Read et al. [19], the choroid was thickest at a point 0.9 mm from the superior temporal point in the macula in children with relatively normal sight (with a refractive error of +1.25 to −0.5 diopter), and that the choroid at a temporal point in the macula thickened with age. In a separate study by Ruiz-Moreno et al. [20], the choroid was thickest at the temporal point of the macula in children with a refractive error of +3.75 to −5.25 diopter, and thinned with age, while the macular point was thickest in adults. In a study by Park and Oh [11], the temporal choroid was significantly thicker than the nasal choroid in healthy Korean children, whereas the superior choroid was significantly thicker than the inferior choroid. In addition, subfoveal choroidal thickness was negatively correlated with age [11]. Together, these studies indicate that choroidal thickness changes according to measurement retinal points and age. In this study, choroidal thickness differed depending on the position of the measurement points and refractive errors. As all subjects in this study were in the same age group, we conclude that choroidal thickness changes according to both retinal measurement points and refractive errors.
The exact mechanism of the changes in choroidal thickness according to the refractive error is not yet fully understood, particularly in children, but this cannot be fully explained simply by a passive increase due to the growth of the eyes. Other studies in children have found that the choroid continuously thickens with age and plateaus in adolescence [19]. According to Troilo et al. [13], thickening of the choroid during normal eye growth in primates is a means of controlling ocular growth as a mechanical buffer that prohibiting axial growth or the diffusion of various growth factors. In particular, the choroid is known to play an important role in the provision of oxygen and nutrients to the external retinal layer [21]. Thus, physiological demands can lead to changes in choroidal conditions. In hyperopia, the energy demand is thought to be high in the macular area to enable rapid axial growth, and the choroid may be thickest in this area to prevent substances required for metabolism from penetrating the choroid too quickly. In emmetropia and myopia, the temporally thickened choroid may function as a buffer to continuous ocular growth in the temporal direction of the macula [13].
Consistent with this hypothesis, we found that in hyperopic eyes, the horizontally located choroid around the fovea was significantly thicker than in emmetropia and the vertically located choroid around the fovea in emmetropia was significantly thicker than in myopia. The choroid at all points besides T3 was thicker in hyperopic than in myopic eyes. The choroid was thinnest at N3, regardless of refractive error. In a previous study of healthy Korean children, the shape of the perifoveal choroid is more likely a regular hexahedron [11]. In addition, data from this study indicated that changes in the shape of the choroid characterized by progressive thinning of the perifoveal choroid may occur as a consequence of eyeball elongation [11]. Due to differences in choroidal thickness at various retinal points, the authors inferred that horizontal stretching of choroidal tissue may occur primarily in the temporal macula during eyeball elongation [11]. Furthermore, there may be a limit in nasal choroidal elongation, as a smaller amount of tissue might be available in the nasal macula due to the presence of the optic nerve head [11]. Given this observation, we hypothesize that axial growth of the eyes is asymmetrical due to refractive errors, and that horizontal growth may occur first from the nasal to the temporal region, followed by vertical growth.
In terms of choroidal thickness according to axial length, the mean axial lengths were 21.54, 23.16, and 24.32 mm in the hyperopia, emmetropia, and myopia groups, respectively; in addition, there was a significant decrease in the subfoveal choroidal thickness. As such, the choroidal thickness seems also to be affected by the axial length, as shown in multiple studies [101522].
This study was limited by its cross-sectional design, as changes over time could not be investigated. Thus, future longitudinal studies are needed to measure changes over time to determine whether changes in the choroid occur due to refractive power. Changes in choroidal thickness in children should also be investigated in different age groups through adolescence, apart from refractive error. Another limitation is the magnification error of Spectralis OCT induced by corneal curvature. In this study, we used the default, standard K value of 7.7 mm when OCT images were taken, although the average K in this study was 7.87. The resulting error in distance measurement parallel to the retinal surface was 1.36% considering that an error of 0.8% each millimeter was noted by the Spectralis software.
In conclusion, choroidal thickness in children with hyperopia was significantly greater than in children with emmetropia or myopia. Thickness also differed according to refractive error, axial length, and location. The fovea in hyperopia and the temporal area in myopia showed the thickest choroid. The choroid in the nasal area of the macula was thinner than the choroid at other areas in all eyes.
Acknowledgements
The authors wish to acknowledge Cheil Eye Hospital and staff involved in the study for their assistance.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.