Recent advances in high-resolution optical coherence tomography (OCT) have helped to reveal the pathogenesis of various vitreomacular pathologies [
1,
2,
3,
4,
5,
6,
7]. Reassessment of macular diseases such as lamellar hole (LH) or macular pseudohole (MPH) with spectral domain (SD)-OCT led to the discovery of distinct epiretinal proliferations with features different from those of the conventional epiretinal membrane (ERM) [
2,
8,
9,
10,
11,
12,
13,
14,
15]. These structures have been referred to using several unestablished terms such as thick ERM [
8], dense non-tractional ERM [
9], thicker ERM [
11], or LH associated retinal proliferation [
13].
This distinct proliferation appears to present properties dissimilar from conventional ERM. Since it was first described by Witkin et al. [
8], this phenomenon has been analyzed in several studies. Parolini et al. [
9] performed a clinicopathological case series study and conducted a histological comparison between dense ERM and conventional tractional ERM. Pang et al. [
13] analyzed 68 of 2,030 SD-OCT images of this proliferation in a retrospective review; the authors presented its distinctive morphological figures and non-tractional properties, proposing the term lamellar hole-associated epiretinal proliferation (LHEP). More recently, Schumann et al. [
12] and Compera et al. [
15] compared various aspects of this unusual epiretinal proliferation between LH and MPH.
Despite a variety of previous studies on unusual epiretinal proliferation, no consensus has been reached regarding its configuration, intraoperative findings, postoperative prognosis, or origin. The objective of this retrospective review of SD-OCT images was to analyze the incidence, morphology, and clinical prognosis of this unusual proliferation. Based on the potential pathogenesis and origin of this occurrence, we propose the new term “epiretinal proliferation associated with macular hole (EPMH).” In addition, we introduce the “perifoveal crown phenomenon” encountered during vitrectomy when peeling away the EPMH.
Materials and Methods
Definitions
1) Epiretinal proliferation associated with LH or MH
In this case study, we found amorphous and substantial epiretinal proliferation in 16 patients using SD-OCT. The proliferative tissue showed a thick blanket-like morphology with medium reflectivity, covering the edge of the MH or LH. We named this unusual amorphous epiretinal proliferation EPMH (
Fig. 1A-1F).
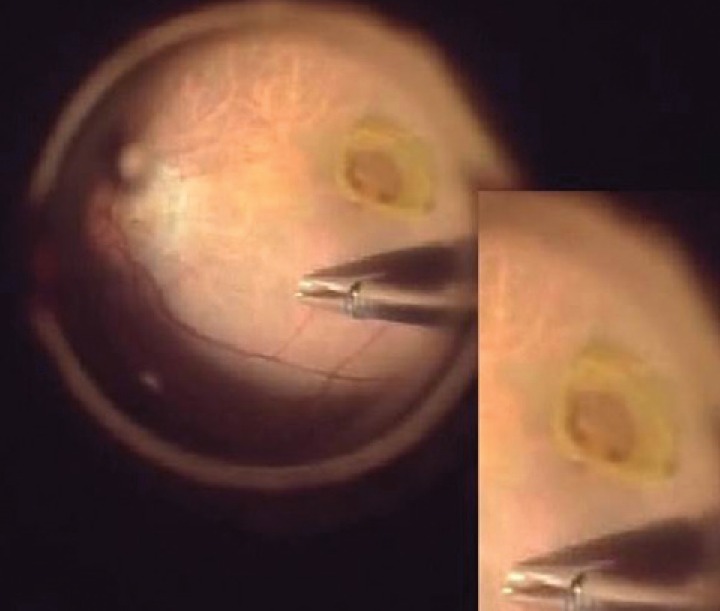
2) Perifoveal crown phenomenon
We centripetally peeled the yellowish epiretinal tissue on the macula that appeared to present as EPMH in the preoperative SD-OCT scan. After peeling the tissue, we observed a floating crown-like yellowish tissue with its base attached to the edge of the margin. We defined this as the perifoveal crown phenomenon (
Fig. 2).
3) Macular hole
For MH diagnosis, we selected patients that fulfilled either one of the two following conditions presented via SD-OCT: (1) full-thickness macular hole (FT-MH) allowing direct connection of the vitreous cavity with bare retinal pigment epithelium (RPE), (2) impending macular hole (I-MH) that showed foveal thinning with evident near-total defect in the outer retina at any point but with no direct connection between the vitreous cavity and bare RPE.
4) Lamellar macular hole
We used previously published SD-OCT criteria [
2,
8] to diagnose LH: (1) foveal thinning with irregular contour, (2) breaks in the inner layers of the fovea, (3) intraretinal split, and (4) absence of a full-thickness foveal defect.
5) Macular pseudohole
MPH was diagnosed according to the following criteria: (1) steepening or verticalization of the foveal edges, (2) increase in perifoveal thickness, and (3) normal central foveal thickness.
Patients
We retrospectively reviewed the medical records of 259 patients diagnosed with MH, LH, or ERM using SD-OCT performed by one surgeon (JS) between January 1, 2013 and December 31, 2014. Among the 259 patients, 42 were excluded because they had other severe concurrent ocular diseases such as end-stage age-related macular degeneration or proliferative diabetic retinopathy. Five vitrectomized patients were also excluded. The prevalence of each LH, MH (FT-MH and I-MH), MPH, and ERM was analyzed.
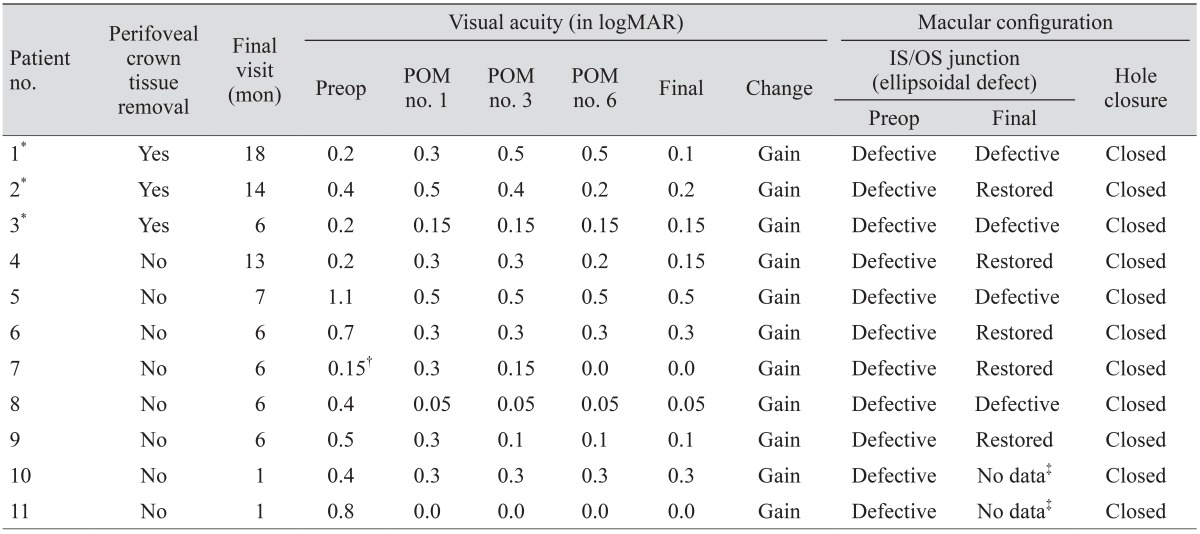
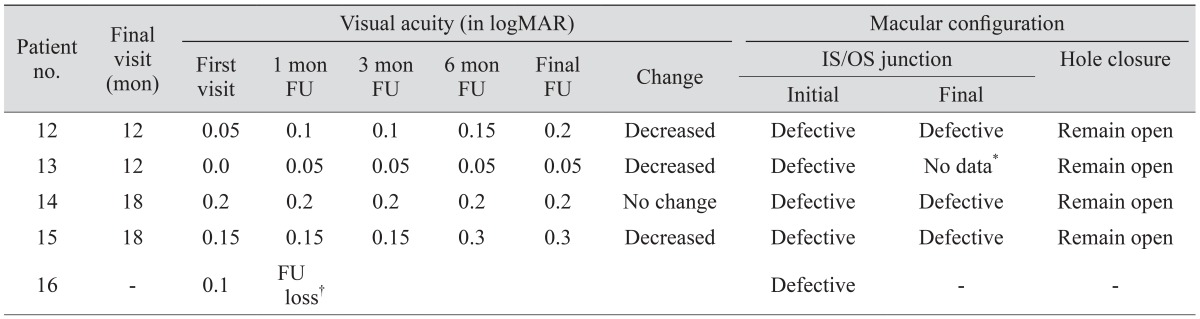
Among the 212 patients, the eye morphology of 16 patients with EPMH was analyzed and clinically followed up. Of these 16 patients, 11 with EPMH (three with FT-MH, four with I-MH, and four with LH) underwent standard vitrectomy. Surgical indications comprised one of the following: (1) FT-MH or I-MH, (2) LH with inner/outer segment (IS/OS) junction disruption and reduced visual acuity 0.2 (in logarithm of the minimal angle of resolution [logMAR] ) or worse, and (3) subjective visual discomfort including metamorphopsia. The other five patients (four with LH and one with I-MH) were followed up periodically without surgical intervention because they did not fulfill any of these indications. One patient with I-MH (no. 16) was lost during follow-up.
Surgical procedure
A 23-gauge transconjunctival suture-less vitrectomy was performed by one surgeon (JS). Concurrent ERM was carefully removed using retinal forceps. A yellowish tissue covering the central macula was identified and presented as EPMH in the preoperative SD-OCT. The yellowish tissue was gently peeled in the centripetal direction using a soft-tip cannula or internal limiting membrane (ILM) forceps. When peeling the tissue, a crown-like structure appeared around the fovea. We named this perifoveal crown tissue (
Fig. 2).
Indocyanine green (ICG)-assisted ILM peeling was performed in all but one patient. ICG was not applied in one patient (no. 2) due to a well-differentiated ILM. Redundant perifoveal crown tissues were trimmed only when there was dehiscence within the flapping yellowish tissue. In three patients (no. 1, 2, and 3), these torn perifoveal crown tissues were removed with retinal scissors, while the tissue was preserved in the other eight patients. Particular care was taken to minimize unnecessary traction on the fovea. A perf luoropropane gas tamponade was performed in three patients (no. 5, 9, and 10), while an air tamponade was performed in the other eight patients. Surgeries were completed without any complications.
Visual acuity and macular configuration follow-up
Visual acuity and macular configuration were recorded throughout the clinical visits of the 16 patients with EPMH. One patient (no. 16) was lost after the initial visit. Visual acuity was measured in logMAR units. Medial opacity was not observed in any of the other 15 patients during follow-up.
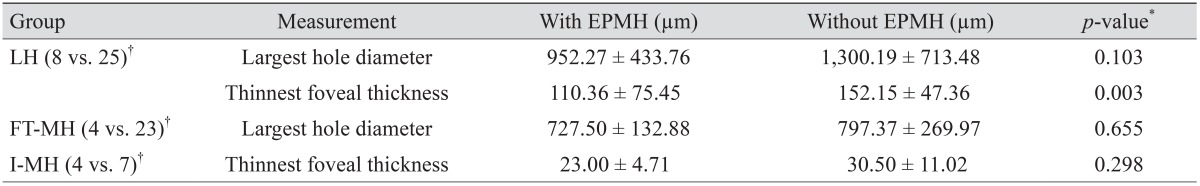
Macular configuration was recorded at every visit using SD-OCT (Cirrus, Carl Zeiss Meditec, Dublin, CA, USA; Spectralis, Heidelberg Engineering, Heidelberg, Germany). Volumes of B-scan images were extracted from Heidelberg software (Heidelberg Viewer Module 5.6.4) and saved as JPG files. The saved images were analyzed by two physicians (GS and SL) and confirmed by a senior grader (JS). The largest hole diameter (for FT-MH and LH) and the thinnest foveal thickness (for I-MH and LH) were measured with equipped caliper software, and IS/OS junction defects and hole closures post-surgery were analyzed. Eyes with FT-MH or I-MH were regarded to have inherent IS/OS junction disruption. Eyes with LH were considered to have defective IS/OS junction when ellipsoidal defect or disconnection was observed (bold triangle) (
Fig. 1).
Specimen preparation
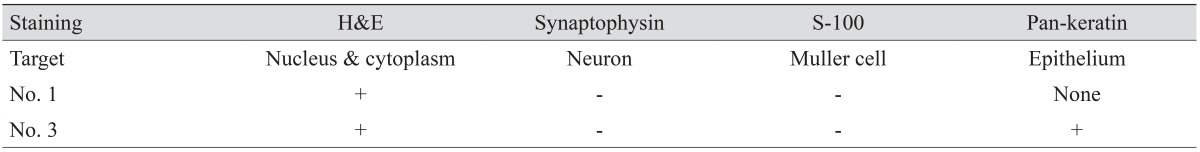
Perifoveal proliferative tissue was removed from the eyes of three patients (no. 1, 2, and 3). Special care was taken to acquire only the yellowish proliferative tissue. Each tissue specimen was fixed in 10% buffered formalin and sent to another affiliation for histological analysis [
2]. Each tissue was then embedded in paraffin and stained with hematoxylin and eosin. Automated immunocytochemical staining was performed using the Bond-max system (Leica Microsystems, Bannockburn, IL, USA). Briefly, all steps were performed using the manufacturer's instructions in the following order: deparaffinization; heat-induced epitope retrieval (antigen unmasking); peroxide block; incubation with synaptophysin (1 : 400, SY38; Dako, Carpinteria, CA, USA), S-100 protein (1 : 500, rabbit polyclonal; Dako), and pan-keratin (1 : 100, AE1/AE3; Novocastra, Newcastle, UK) primary antibodies; color development with 3,3′-diaminobenzidine tetrahydrochloride chromogen; hematoxylin counterstaining; and mounting of the slides. Normal human serum served as a negative control. Samples were considered positive for synaptophysin, S-100 protein, and pan-keratin if they exhibited any degree of cytoplasmic staining. For photodocumentation, we used a digital camera to image the specimens at ×200 magnification (E1000; Nikon, Tokyo, Japan). Synaptophysin was used to mark cells of neuronal origin, S-100 protein for Müller cells, and pan-keratin for cells of epithelial origin.
Ethics statement
This study was approved by the institutional review board of HanGil Eye Hospital. Informed consent was obtained from all 16 patients with EPMH. This study adhered to the tenets of the Declaration of Helsinki.
Discussion
The unusual epiretinal proliferation found in MH or LH is now accepted as a distinct clinical entity from conventional ERMs [
13]. Recent studies have observed some common morphological configurations of this unusual proliferation [
12,
14,
15]. It is most frequently observed in eyes with LH [
8,
11,
13,
16] and is associated with a large hole diameter and a thin fovea [
14]. It does not appear to occur in eyes with an intact IS/OS junction, such as those with MPH [
12,
15]. This study verified these previous observations with the exception of the association of EPML with large LH diameter. All 16 patients with EPMH exhibited concurrent ERM. Eight patents with LH associated with EPMH had a significantly thinner central fovea than that of 25 patients without EPMH; however, there was no significant difference in LH diameter between these patients. IS/OS junctional disruption was observed in all 16 patients with EPMH.
We noted some new findings regarding this clinical entity. First, a certain number of EPMH cases were observed not only in eyes with LH, but also in eyes with FT-MH or I-MH. Recent studies have mainly reported this unusual proliferation in patients with LH [
8,
11,
13,
16]. Pang et al. [
13] reported that 60 of 68 cases (88.2%) of LHEP occurred in eyes with LH, while the remaining 11.8% of cases were found in eyes with FT-MH. In our study, eight of the 16 patients with LH (50%) exhibited EPMH, while it occurred in eight of the eyes with FT-MH (25%) or I-MH (25%). Although we analyzed a smaller number of cases, EPMH was not observed as frequently in LH cases as was previously reported.
Second, we observed the perifoveal crown phenomenon in all patients with EPMH when peeling this unusual proliferative tissue. Shiraga et al. [
16] suggested that preserving the “thick ERM with macular pigment” yields better clinical outcomes than removing it when peeling ILM in vitrectomy. They presented a photograph of an inverted “thick ERM” tissue flapping over the LH after peeling it centripetally to the edge of the hole. Compera et al. [
15] also described LHEP as a dense yellowish tissue of fluffy consistency, making it difficult to grasp. Although these authors used a different term, we infer that their descriptions represent other examples of what we defined as the perifoveal crown phenomenon. Third, we observed positive pan-keratin staining in one patient. Pan-cytokeratin is a monoclonal antibody cocktail that demonstrates a broad spectrum of reactivity and stains the cytokeratins of virtually all human epithelia. RPE is the only layer of the retina that presents positivity to pan-keratin. Staining the EPMH with synaptophysin or S-100, which target neurons and Müller cells, respectively, presented negative results in two consecutive tissue specimens.
Fourth, EPMH appears to be continuous to the defective point of the IS/OS junction (
Fig. 3). IS/OS disruptions have been frequently reported in eyes with LH in previous SD-OCT based studies [
10,
13,
14]. In eyes with LH, EPMH has presented a higher frequency of IS/OS disruption than in eyes without EPMH [
14]. Pang et al. [
13] demonstrated contiguity of the LHEP with the middle retinal layer. In the present study, anatomical continuity was observed through consecutive SD-OCT section images of the 16 patients with EPMH. By drawing the boundary of the area with the same optical density, we verified that EPMH extends to the IS/OS disruption, beyond the middle retinal layer. Based on these findings, we suggest that RPE can migrate through the defective outer retina and proliferate into EPMH. In addition, the IS/OS junction disruption could have served as a channel through which RPE could migrate. In this study, all 16 patients with EPMH showed IS/OS disruption.
There have been various attempts to explain the pathogenesis of this unusual epiretinal proliferation. Parolini et al. [
9] suggested premacular vitreous remodeling as a potential causative mechanism and presented positive staining results of “dense membrane” for glial fibrillary acid protein (GFAP) and hyalocyte markers (CD45 and CD64) as histologic evidence. Although that group reported only a few positive staining results of dense membrane for cellular retinaldehyde binding protein, which targets both glial cells and RPE cells, they suspected hyalocytes as the causative agent instead of RPE cells. Shiraga et al. [
16] speculated that a portion of the retinal tissue would migrate along the posterior hyaloid face, forming ERM with macular pigments. Pang et al. [
13] hypothesized the possibility of Müller cell proliferation with SD-OCT based anatomical assessment of LHEP. Recently, Compera et al. [
15] presented a similar opinion to that of Parolini et al. [
9], that LHEP appears to occur due to vitreous reaction.
Some authors have suggested gliosis as the potential causative mechanism of the formation of EPMH. Positive GFAP staining against EPMH tissue was observed in several previous studies [
9,
15]. However, the authors did not appear to distinguish EPMH from ILM or ERM and instead performed a combined fixation process. ILM specimens are composed of a variety of cells including hyalocytes, glial cells, RPE, fibrocytes, and myofibrocytes [
17,
18,
19]. Therefore, their positive staining results do not appear to be limited only to EPMH. In our study, we harvested only a portion of the yellowish pigment-rich tissue when we noted dehiscence within the fluttering perifoveal crown tissue. Since the tissue acquisition was performed after ICG-assisted ILM peeling, there should have been a lower risk of target tissue contamination with ILM components. Therefore, we believe that more accurate histological analysis was possible with our harvesting technique. Furthermore, in immunohistochemical staining, none of our tissue specimens presented a positive reaction to S-100 protein, a calcium-binding protein that labels the cytoplasm of Müller cells [
20], the principal glial cells of the retina. The negative results for S-100 do not support the hypothesis of Müller cell gliosis as the causative mechanism of EPMH.
Removal of EPMH is not recommended. In our study, three of four patients with LH, whose EPMH was preserved, presented a stable natural clinical course throughout the 1-year follow-up period. In contrast, one patient in whom a greater amount of EPMH was removed than intended presented initial reduction and delayed recovery in visual acuity. In previous studies, EPMH showed morphological stability in more than 90% of eyes without surgical intervention [
11,
13], and removing EPMH may have led to development of a FT-MH [
8,
9,
13]. Pang et al. [
13] hypothesized that removing this tissue would draw the central plug out of the structurally compromised macula that was stabilized by the tissue. In our experiences, trimming a portion of the perifoveal crown tissues did not appear to decrease visual acuity or structural recovery. However, considering the potential complications, preserving the tissue or at least leaving the stump may comprise a better strategy when performing vitrectomy.
There are a few limitations to this study. First, it was a retrospective review of 212 patients at a single institute; this sample size was insufficient to establish statistical significance. Additional cases are required to elucidate detailed characteristics and the potential origins of this unusual proliferative tissue. Second, the positive staining result of pan-keratin was noted in only a single case. The scarcity of histological analyses in this study is due to our speculation that preserving the EPMH would be better for visual acuity and macula stabilization. We did not suspect RPE as a potential origin of proliferation when we harvested the first specimen. As this study progressed, analyses of SD-OCT images led us to consider RPE as a possible origin and to add pan-keratin antibody to the second immunocytochemical staining. Third, using S-100 instead of GFAP could be another limitation in this histological analysis. The upregulation of GFAP is the most sensitive non-specific response to retinal disease [
21], and GFAP has been used as universal marker of Müller cell gliosis [
22,
23]. However, S-100 protein has also been used as a Müller cell marker [
24,
25,
26], and two consecutive negative staining results of S-100 should not be disregarded. Further histological analysis is required to provide further information on the potential causes of EPMH formation.
In conclusion, we suggest that EPMH could be the result of RPE proliferation that migrated through IS/OS junction defects. EPMH appears to occur mainly in eyes with LH; however, it can also be present in eyes with other vitreomacular pathologies such as FT-MH or I-MH. Perifoveal crown phenomenon can be regarded as an intraoperative characteristic. Additional histological analysis utilizing more accurate harvesting techniques is necessary to improve our understanding of this phenomenon.









 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print