 |
 |
| Korean J Ophthalmol > Volume 30(5); 2016 > Article |
Abstract
Purpose
To investigate optic nerve head size and retinal nerve fiber layer (RNFL) thickness according to refractive status and axial length.
Methods
In a cross-sectional study, 252 eyes of 252 healthy volunteers underwent ocular biometry measurement as well as optic nerve head and RNFL imaging by spectral-domain optical coherence tomography. Correlation and linear regression analyses were performed for all subjects. The magnification effect was adjusted by the modified axial length method.
Results
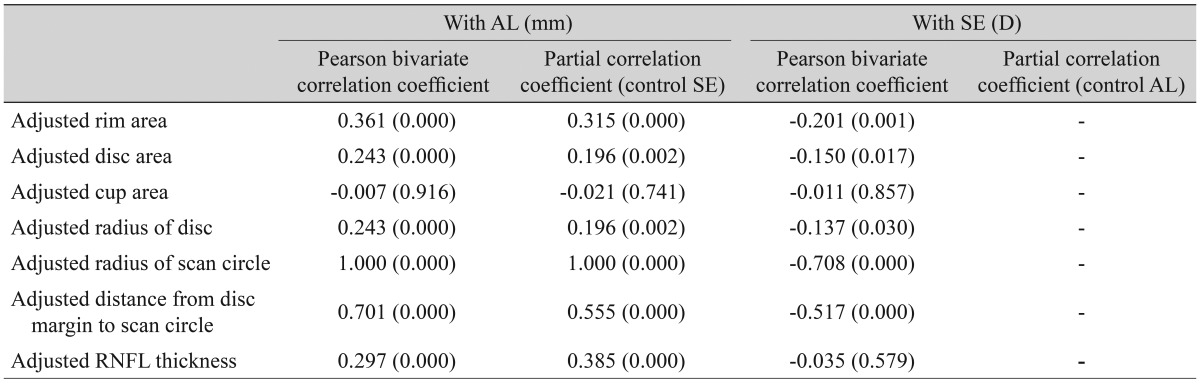
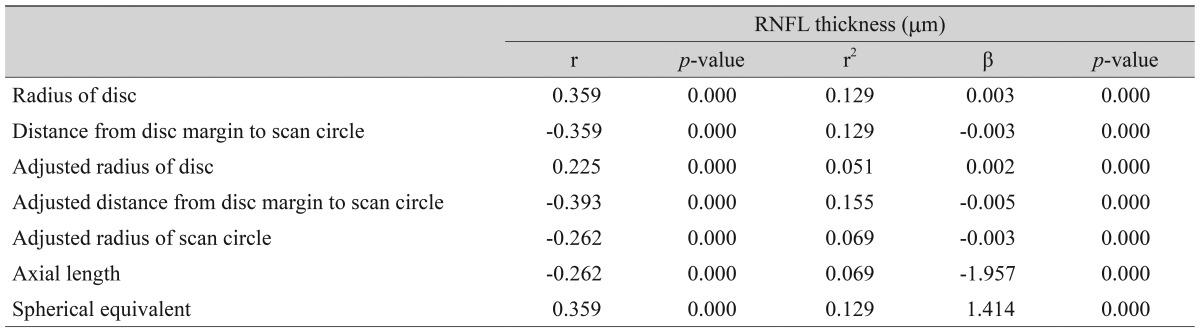
Disc area and spherical equivalent were positively correlated (r = 0.225, r2 = 0.051, p = 0.000). RNFL thickness showed significant correlations with spherical equivalent (r = 0.359, r2 = 0.129, p = 0.000), axial length (r = -0.262, r2 = 0.069, p = 0.000), disc radius (r = 0.359, r2 = 0.129, p = 0.000), and radius of the scan circle (r = -0.262, r2 = 0.069, p = 0.000). After adjustment for the magnification effect, those relationships were reversed; RNFL thickness showed negative correlation with spherical equivalent and disc radius, and positive correlation with axial length and radius of the scan circle. The distance between the disc margin and the scan circle was closely correlated with RNFL thickness (r = -0.359, r2 = 0.129, p = 0.000), which showed a negative correlation with axial length (r = -0.262, r2 = 0.069, p = 0.000).
Conclusions
Optic disc radius and RNFL thickness decreased in more severely myopic eyes, but they increased after adjustment for magnification effect. The error due to the magnification effect and optic nerve head size difference might be factors that should be considered when interpreting optical coherence tomography results.
Morphologic evaluation of the optic nerve head (ONH) is an essential step in the proper diagnosis of optic nerve diseases, including glaucoma. For example, the ONH of primary open-angle glaucoma patients is characterized by a large and deep cup with a narrow neuroretinal rim, resulting from the loss of ganglion cell axons [1,2]. Unfortunately, the size and shape of the ONH and cup not only vary with specific optic nerve diseases, but also differ widely in healthy eyes by ethnicity, sex, and refractive status; differences exist even between eyes of the same individual [3,4,5,6,7]. This large variation in healthy ONH appearance makes precise detection of ONH morphologic abnormalities very difficult despite recently developed advanced imaging systems such as Heidelberg retina tomography and optical coherence tomography (OCT).
Notably, myopia has been widely reported to affect the size and shape of the optic disc and peripapillary retinal nerve fiber layer (RNFL) [8,9,10,11]. Diagnosis of glaucoma in myopic patients is thus very challenging. Thorough and accurate understanding of the relationship between myopia and the anatomic structures of the ONH and RNFL is important, particularly in light of the two to three times greater risk of glaucoma in myopic individuals compared with nonmyopic individuals [12,13]. However, the influence of myopia on the shape and size of the ONH and peripapillary RNFL is still uncertain [8,11,14,15,16]. Moreover, most of the previous studies on ONH and RNFL appearance in myopia were conducted with subject groups heterogeneous in age, sex, and ethnicity. As emphasized above, factors such as age, sex, and ethnicity are known to affect ONH and peripapillary RNFL morphology. This signifies the importance of subject homogeneity with regard to age, sex, and ethnicity in any effort to elucidate the relationship between myopia and ONH/RNFL morphology. In this regard, for the purposes of the present study, healthy volunteers matched by age (young), sex (male), and ethnicity (Korean) were recruited. Data regarding ONH and RNFL structure and refractive errors were collected and analyzed in relation to the degree of myopia.
Soldiers stationed in Gyeonggi province were invited to participate in the study, which was conducted between December 2008 and April 2009. The study met the ethical standards of the Declaration of Helsinki and was approved by the Armed Forces Capital Hospital institutional review board. In addition, informed consent was obtained from each participant, and individuals with any abnormal ocular findings or history of certain diseases were excluded. The specific exclusion criteria were as follows: (1) ocular hypertension (IOP >21 mmHg) or glaucoma; (2) evidence of reproducible visual field abnormality (defined as pattern standard deviation significant at p < 5% level, abnormal glaucoma hemifield test result, or any other pattern of loss consistent with ocular disease) in either eye; (3) history of ocular surgery; (4) best-corrected visual acuity worse than 20 / 32 on Early Treatment of Diabetic Retinopathy Study scale; (5) evidence of vitreoretinal disease; (6) evidence of optic nerve or RNFL abnormality; and (7) history of diabetes or other systemic disease.
All subjects underwent comprehensive ophthalmologic examinations on both eyes; these examinations included best-corrected visual acuity, intraocular pressure with Goldmann applanation tonometry, automated refraction (RK-F1; Topcon, Tokyo, Japan), axial length (IOLMaster; Carl Zeiss Meditec, Dublin, CA, USA), slit-lamp examination, red-free fundus photography (CF-60UVi; Canon, Tokyo, Japan) with mydriasis, standard automated perimetry (Swedish Interactive Threshold Algorithm standard C24-2 program, Humphrey Field Analyzer II 750; Carl Zeiss Meditec), ONH parameter measurement (rim area, disc area, average cup-to-disc [C/D] ratio, vertical C/D ratio, and cup volume), and peripapillary RNFL thickness measurement by spectral-domain OCT (Cirrus OCT, Carl Zeiss Meditec). The data for one randomly selected eye were selected for analysis.
The refractive error was measured five times by autorefractometry (R-F10, Canon) without cycloplegia, and the result was subsequently converted to spherical equivalent. The average of three median values, after discarding the upper and lower values, was used in the analysis. The axial length was measured five times by partial coherence interferometry (IOLMaster, Carl Zeiss Meditec), and the average was calculated using the same process as that used for refractive error.
The ONH parameters and peripapillary RNFL thicknesses were measured after the red-free fundus photography by spectral-domain OCT with the optic disc cube 200 × 200 scan protocol under pupil dilatation and dim illumination by two expert examiners. Scanned images of signal strength lower than 8 were discarded. Also excluded were individuals with an extent of peripapillary atrophy that expanded across the 3.46 mm scan circle centered on the optic disc. Clock-hour RNFL thickness was recorded based on the right-eye orientation. The optic disc margin measured by spectral-domain OCT was sometimes different from the actual disc margin because spectral-domain OCT determines disc margin based on the retinal pigment epithelium. However, it did not have a significant impact on the analysis, so we included all data if peripapillary atrophy did not expand past the scan circle radius.
The ONH average radius was calculated from the OCT-measured disc area by the following equations: Therefore,
With this method, the distance from the disc margin to the scan circle (distance = radius of scan circle - radius of disc), which is suspected to influence the OCT peripapillary RNFL thickness measurement, was determined.
Adjustment for the ocular magnification effect was performed in the same way as in our previous work with the modified axial length method [17]. The relationship between the size of an object on the fundus as measured on a fundus photograph and the actual size of the object on the fundus can be expressed as t = p · q · s, where "t" is the actual size, "s" is the size measured on the fundus photograph, "p" is the magnification factor related to the camera, and "q" is the magnification factor related to the eye [18]. The magnification factor related to the fundus camera, "p," can be expressed as a constant of 3.382 in the telecentric system of Stratus a nd Cirrus OCT [19]. The ocular magnification factor related to the eye, "q," was calculated by the modified axial length method proposed by Bennett et al. [20] [q = 0.01306 · {axial length (mm) - 1.82}].
Adjustment for average RNFL thickness was performed in the same way as in our previous work [17]. It was presumed that the same number of retinal nerve fibers crossed the scan circle of a 1.73 mm radius and the magnified scan circle at the same time. Accordingly, the cylindrical cross-sectional RNFL area under the 1.73 mm radius scan circle and the magnified scan circle should be the same. The radius of the magnified scan circle was calculated using the aforementioned modified axial length method. From this value, the adjusted average RNFL thickness under the 1.73 mm scan circle was calculated using the equation (r, radius of scan circle = 1.73 mm; r', magnified radius of scan circle).
Therefore,
Statistical analysis was carried out using the SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). The spectral-domain OCT-measured ONH parameters and the calculated mean radius of the disc were compared. Bivariate and partial correlation analyses were performed to investigate the relationship between axial length or spherical equivalent and ONH parameters or peripapillary RNFL thickness. In addition, a partial correlation analysis for the same variables, which controlled for spherical equivalent, was performed to investigate the shear influence of axial length on ONH parameters and peripapillary RNFL thickness, apart from the effect of refractive status. Correlation and linear regression analyses of the ONH parameters, peripapillary RNFL thickness, calculated disc radius, distance from disc margin to scan circle, axial length, and refractive error were performed for all subjects. The p-values less than 0.01 were considered statistically significant.
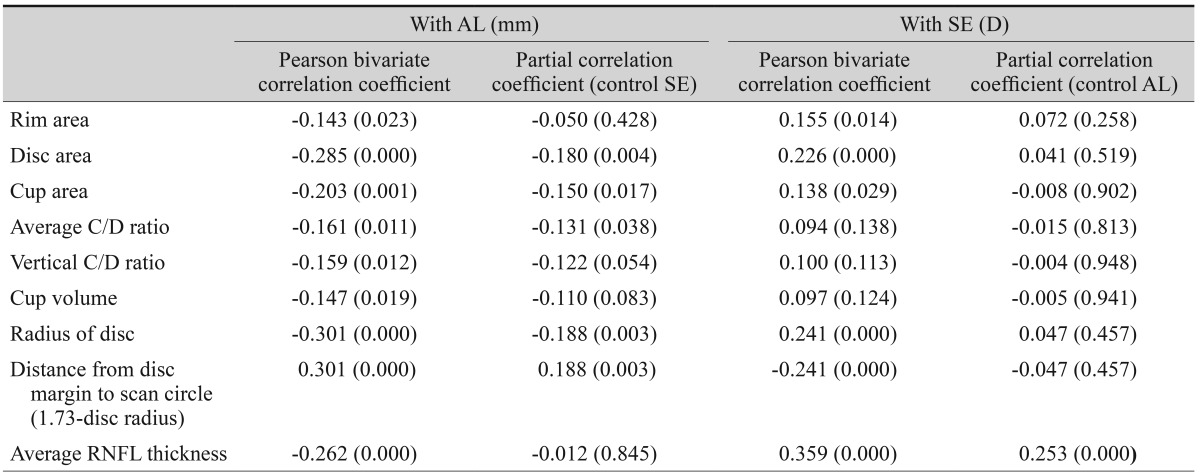
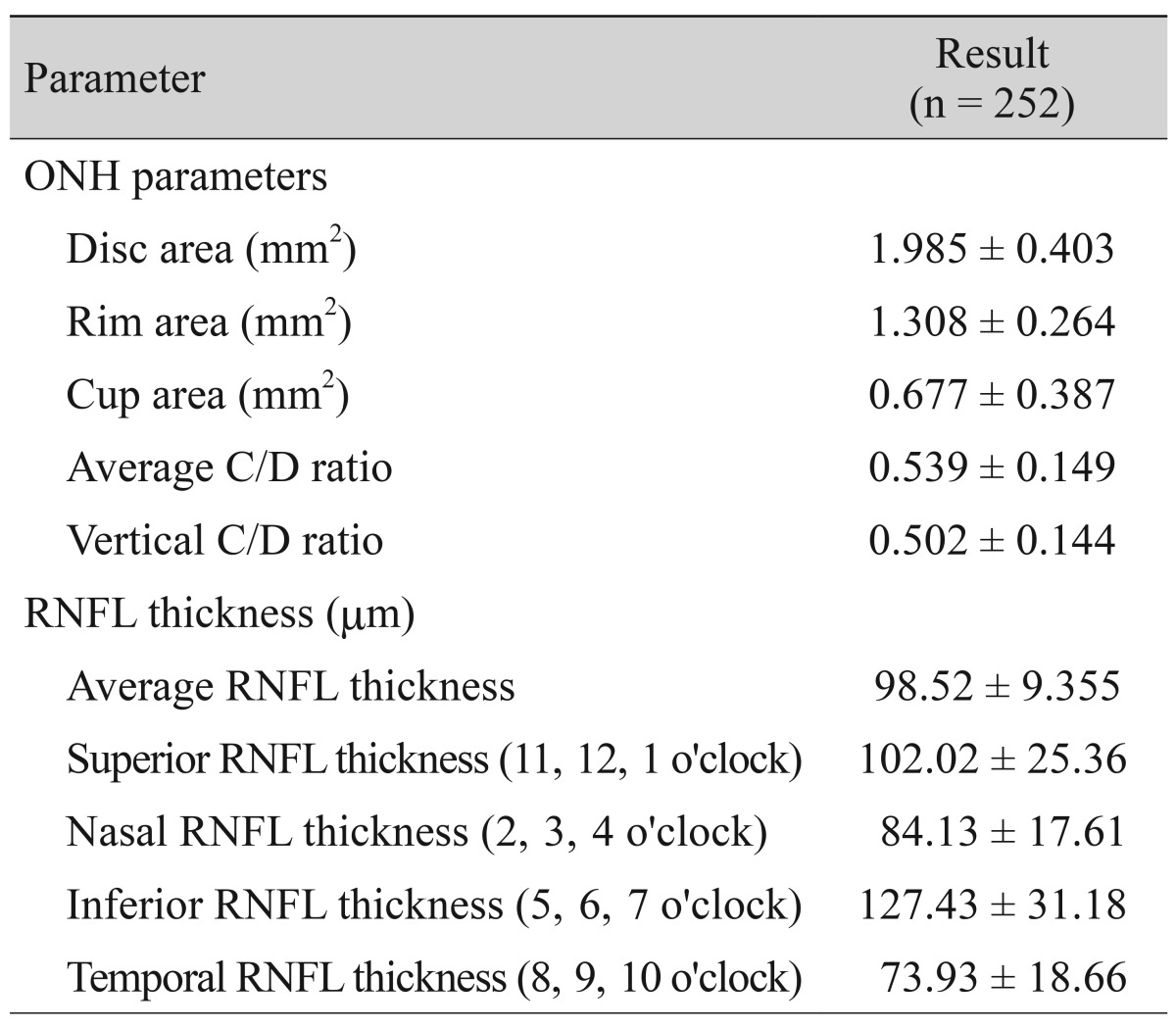
A total of 258 subjects were enrolled in this study. Among them, six were excluded because of extended peripapillary atrophy across the 1.73 mm radius scan circle or unacceptable OCT scans, leaving 252 eyes of 252 subjects for further analysis. The mean age of the 252 subjects was 21.06 ± 1.64 years (19 to 26). The average axial length was 24.74 ± 1.25 mm (21.38 to 28.59), and the mean refractive error was -2.51 ± 2.37 (-11.0 to +4.13) diopters (D). The ONH parameters and peripapillary RNFL thicknesses measured by spectral-domain OCT are listed in Table 1. The average disc area for the entire subject group was 1.985 ± 0.403 mm2, the r im area was 1.308 ± 0.264 mm2, and the average C/D ratio was 0.539 ± 0.149. A correlation analysis of the axial length with disc area, rim area, cup area, C/D ratio, and peripapillary RNFL thickness showed negative results. The distance from the disc margin to the scan circle (1.73-disc radius) showed positive correlation with axial length. A partial correlation analysis of the same variables, which controlled for the spherical equivalent, showed negative correlation with axial length, whereas the distance from the disc margin to the scan circle showed a positive correlation. A correlation analysis of the spherical equivalent with the disc area, rim area, cup area, C/D ratio, and RNFL thickness showed positive correlations, while that of the spherical equivalent with the distance from the disc margin to the scan circle showed a negative correlation. A partial correlation analysis that controlled for the axial length also revealed positive correlations of the spherical equivalent with the ONH parameters and peripapillary RNFL thickness and a negative correlation with the distance from the disc margin to the scan circle (Table 2). The adjusted values of the disc area, rim a rea, radius of the scan c ircle, a nd a verage RNFL thickness all showed significant positive correlation with the axial length, regardless of the spherical equivalent. Adjusted cup area correlated negatively with the axial length, but this was without statistical significance. All of the adjusted parameters showed negative correlation with the spherical equivalent (Table 3). The same relationships were observed in the results of a linear regression analysis. Specifically, as the axial length increased, the disc area, disc radius, and average RNFL thickness decreased, whereas the distance from the disc margin to the scan circle (of 1.73-mm radius) increased significantly. Also, as the spherical equivalent increased, the disc area, disc radius, and average RNFL thickness increased, and the distance from the disc margin to the scan circle decreased significantly. After adjustment for the ocular magnification effect, as the axial length increased, the disc area, disc radius, and average RNFL thickness decreased, and the distance from the disc margin to the scan circle increased significantly; as the spherical equivalent increased, the disc area, disc radius, average RNFL thickness, and the distance from the disc margin to the scan circle decreased (Figs. 1A-1F and 2A-2F). The measured RNFL thickness without the adjusted ocular magnification effect was analyzed using correlation and simple linear regression analyses to investigate the relationship between disc size and scan circle radius. It was found that the measured RNFL thickness was larger in the eyes with a longer disc radius both before and after adjustment for the ocular magnification effect. In contrast, with the adjusted scan circle, the measured RNFL thickness was smaller in the eyes with a longer distance from the disc margin to the scan circle. Finally, the eyes with the longer axial length and larger myopic refractive error showed smaller peripapillary RNFL thickness as measured by spectral-domain OCT (Table 4).
Many studies have suggested that optic disc size is influenced by axial length and refractive error [5,8,9,10,11,16,21]; however, the results of these studies are conflicting. For example, Cheung et al. [22] reported that the optic disc is small in myopic eyes, whereas Jonas [23] claimed that it was large. In the present study, optic disc size, axial length, and refractive error were measured in healthy volunteers, and the results were subjected to correlation analyses. For these purposes, spectral-domain OCT was employed, and the ocular magnification effect was corrected using the modified axial length method of Bennett et al. [20]. Although the accuracy of this correction method is uncertain, it is known to be both simpler and more accurate than other modalities currently available [24]; in fact, several recent studies have used it to correct for the ocular magnification effect of ophthalmologic imaging devices [16,17,21]. In any case, the results of the present study were similar to those obtained in prior studies reported by Leung et al. [16] and Savini et al. [21], who utilized the same correction method for the ocular magnification effect. Specifically, after correction for the ocular magnification effect, the optic disc size and peripapillary RNFL thickness were both larger in more severely myopic eyes. These findings are highly consistent with those of the histological study carried out by Jonas et al. [25].
Considering both the homogeneous characteristics of the subjects enrolled in this study and the fact that their respective findings did not significantly diverge from those of prior studies on subjects of varying age, sex, and ethnicity, it was concluded that ONH size, peripapillary RNFL thickness, and myopia might be independent of such factors. If this is true, an ophthalmologist, when interpreting data obtained with imaging devices such as OCT or fundus photography, could apply the same considerations with regard to disc size, the effect of ocular magnification, and the degree of myopia for any age, gender, or ethnicity. Nonetheless, in the clinical setting, whereas peripapillary RNFL thickness evaluation provides some of the most important information regarding glaucomatous optic nerve damage, it is impossible to correct for the ocular magnification effect in every OCT scan result. Therefore, the present study analyzed the influence of several parameters on scanned peripapillary RNFL thickness without correction for the ocular magnification effect. According to our study, the adjusted peripapillary RNFL thickness showed a negative correlation with spherical equivalent without statistical significance and a positive correlation with axial length, results similar to those of a previous study [17]. However, the partial correlation analysis of the spherical equivalent with the ocular magnification effect as adjusted for the parameters controlling the axial length could not be completed, because the adjustment for the ocular magnification effect and the partial correlation analysis used the same variables simultaneously. Furthermore, the peripapillary RNFL thickness was larger in cases where the radius of the optic disc was large, the radius of the scan circle was reduced because of the ocular magnification effect, and the distance from the disc margin to the scan circle was short because of a large disc or small scan circle or both. Another interesting finding was that the coefficient of determination of the distance from the disc margin to the scan circle for the peripapillary RNFL thickness was higher than any others. This suggests that peripapillary RNFL thickness is influenced more by the distance from the disc margin to the scan circle than by other factors including the radius of the scan circle, which is the distance from the center of disc to the scan circle. If the same number of nerve fibers enters the eye through the ONH, the RNFL thickness measured at the same location should not differ, regardless of the disc size. Therefore, the correlations of peripapillary RNFL thickness with the distance from the disc margin to the scan circle and with the distance from the disc center to the scan circle should be very similar. However, in this study, the measured peripapillary RNFL thickness was more closely correlated with the distance from the disc margin to the scan circle. The possible explanations of this finding are as follows. First, more redundant non-neural tissues such as glial cells or capillaries could exist within the RNFL adjacent to the disc. However, confirmation of this hypothesis requires additional histological evidence not yet available. Second, the RNFL might contain a larger number of nerve fibers in eyes with a short distance between the disc margin and the scan circle. As noted above, a large disc radius or reduced scan circle radius because of hyperopia could shorten that distance. However, according to the present results, hyperopic eyes had both a short axial length and a small disc radius, suggesting that the small radius of the optic disc might compensate for the decreased radius of the scan circle. Therefore, whether a large optic disc contains more nerve fibers might have a crucial effect on peripapillary RNFL thickness measurement. Verification of this effect will have to await further histological study.
The study was conducted only in a limited sample of healthy young Korean males. Therefore, these features may limit the application of these data to subjects of other age groups or ethnicities.
In conclusion, the peripapillary RNFL thickness was most strongly influenced by the distance from the disc margin to the scan circle. Disc radius and RNFL thickness decreased in more severely myopic eyes, they increased after adjustment for the magnification effect. Based on this, the ONH size and RNFL measurements were influenced by the magnification effect. Although the error by the magnification effect and the ONH size difference were clinically negligible because of the low coefficient of determination and extremely small optic disc size change according to the degree of myopia, they might remain as factors that should be considered.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article were reported.
REFERENCES
2. Lee KH, Park KH, Kim DM, Youn DH. Relationship between optic nerve head parameters of Heidelberg Retina Tomograph and visual field defects in primary open-angle glaucoma. Korean J Ophthalmol 1996;10:24-28.


3. Chi T, Ritch R, Stickler D, et al. Racial differences in optic nerve head parameters. Arch Ophthalmol 1989;107:836-839.


5. Varma R, Tielsch JM, Quigley HA, et al. Race-, age-, gender-, and refractive error-related differences in the normal optic disc. Arch Ophthalmol 1994;112:1068-1076.


6. Tsai CS, Zangwill L, Gonzalez C, et al. Ethnic differences in optic nerve head topography. J Glaucoma 1995;4:248-257.


7. Marsh BC, Cantor LB, WuDunn D, et al. Optic nerve head (ONH) topographic analysis by stratus OCT in normal subjects: correlation to disc size, age, and ethnicity. J Glaucoma 2010;19:310-318.



8. Tomlinson A, Phillips CI. Ratio of optic cup to optic disc: in relation to axial length of eyeball and refraction. Br J Ophthalmol 1969;53:765-768.



9. Jonas JB, Gusek GC, Naumann GO. Optic disk morphometry in high myopia. Graefes Arch Clin Exp Ophthalmol 1988;226:587-590.


10. Hyung SM, Kim DM, Hong C, Youn DH. Optic disc of the myopic eye: relationship between refractive errors and morphometric characteristics. Korean J Ophthalmol 1992;6:32-35.


11. Samarawickrama C, Wang XY, Huynh SC, et al. Effects of refraction and axial length on childhood optic disk parameters measured by optical coherence tomography. Am J Ophthalmol 2007;144:459-461.


12. Hoh ST, Lim MC, Seah SK, et al. Peripapillary retinal nerve fiber layer thickness variations with myopia. Ophthalmology 2006;113:773-777.


13. Melo GB, Libera RD, Barbosa AS, et al. Comparison of optic disk and retinal nerve fiber layer thickness in nonglaucomatous and glaucomatous patients with high myopia. Am J Ophthalmol 2006;142:858-860.


14. Uchida H, Yamamoto T, Araie M, et al. Topographic characteristics of the optic nerve head measured with scanning laser tomography in normal Japanese subjects. Jpn J Ophthalmol 2005;49:469-476.


15. Tay E, Seah SK, Chan SP, et al. Optic disk ovality as an index of tilt and its relationship to myopia and perimetry. Am J Ophthalmol 2005;139:247-252.


16. Leung CK, Cheng AC, Chong KK, et al. Optic disc measurements in myopia with optical coherence tomography and confocal scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci 2007;48:3178-3183.


17. Kang SH, Hong SW, Im SK, et al. Effect of myopia on the thickness of the retinal nerve fiber layer measured by Cirrus HD optical coherence tomography. Invest Ophthalmol Vis Sci 2010;51:4075-4083.


18. Littmann H. Determination of the real size of an object on the fundus of the living eye. Klin Monbl Augenheilkd 1982;180:286-289.


19. Bengtsson B, Krakau CE. Correction of optic disc measurements on fundus photographs. Graefes Arch Clin Exp Ophthalmol 1992;230:24-28.


20. Bennett AG, Rudnicka AR, Edgar DF. Improvements on Littmann's method of determining the size of retinal features by fundus photography. Graefes Arch Clin Exp Ophthalmol 1994;232:361-367.


21. Savini G, Barboni P, Parisi V, Carbonelli M. The influence of axial length on retinal nerve fibre layer thickness and optic-disc size measurements by spectral-domain OCT. Br J Ophthalmol 2012;96:57-61.


22. Cheung CY, Chen D, Wong TY, et al. Determinants of quantitative optic nerve measurements using spectral domain optical coherence tomography in a population-based sample of non-glaucomatous subjects. Invest Ophthalmol Vis Sci 2011;52:9629-9635.


Fig. 1
Linear regression analysis of relationships of optic nerve head parameters, radius of scan circle, and peripapillary retinal nerve fiber layer (RNFL) thickness to axial length before and after adjustment for ocular magnification effect. (A) Rim area, (B) disc area, (C) cup area, (D) radius of disc, (E) disc-scan circle distance, and (F) RNFL thickness.
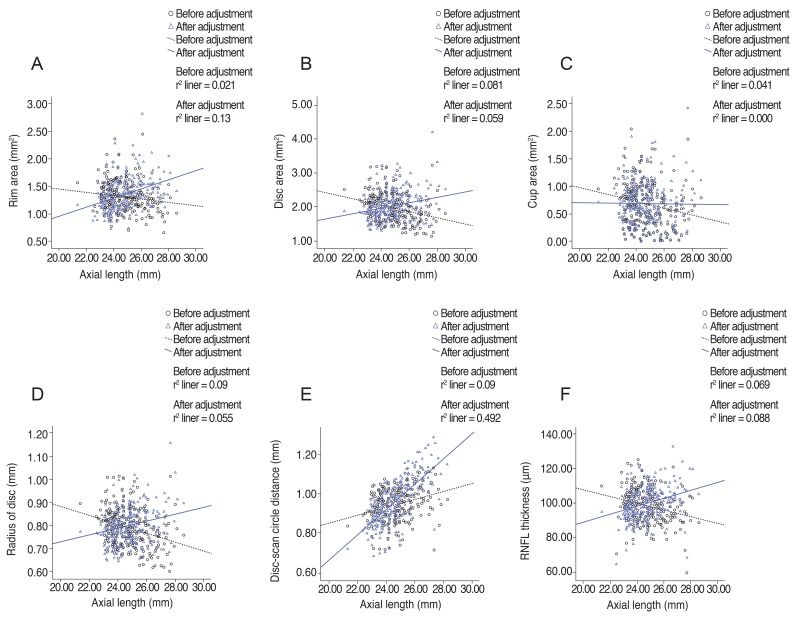
Fig. 2
Linear regression analysis of relationships of optic nerve head parameters, radius of scan circle, and peripapillary retinal nerve fiber layer (RNFL) thickness to spherical equivalent before and after adjustment for ocular magnification effect. (A) Rim area, (B) disc area, (C) cup area, (D) radius of disc, (E) disc-scan circle distance, and (F) RNFL thickness. D = diopters.
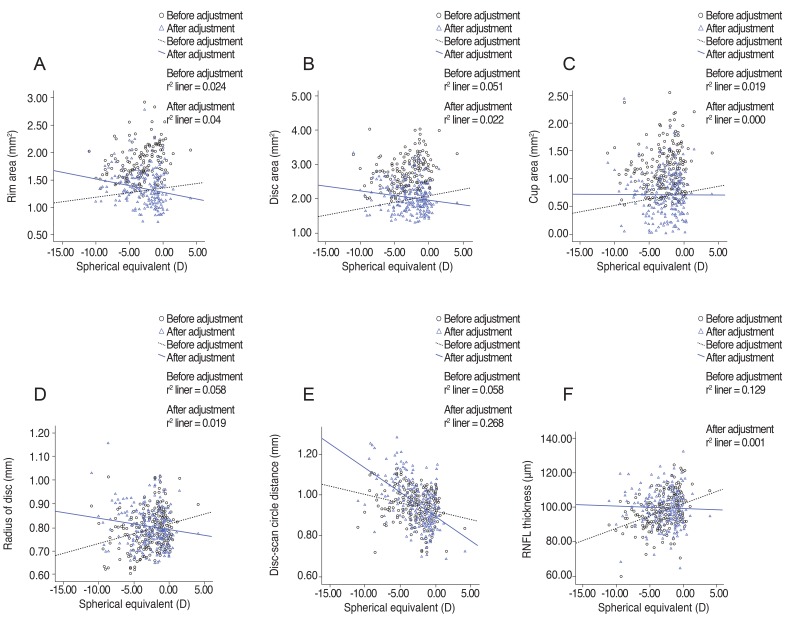
Table 2
Bivariate and partial correlations of AL and SE with disc parameters and peripapillary RNFL thickness before adjustment for ocular magnification effect



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




