|
 |
| Korean J Ophthalmol > Volume 30(4); 2016 > Article |
Abstract
Purpose
To evaluate 12-month outcomes of anti-vascular endothelial growth factor (VEGF) therapy for polypoidal choroidal vasculopathy (PCV) with grape-like polyp clusters.
Methods
This retrospective observational study included 23 eyes of 23 patients who were newly diagnosed with PCV with grape-like polyp clusters, and who were subsequently treated with anti-VEGF monotherapy. The study compares the best-corrected visual acuity (BCVA) of the patients at diagnosis, at 3 months, and at 12 months after diagnosis. In addition, 12-month changes in BCVA values were compared between cases with subfoveal or juxtafoveal polyps and cases with extrafoveal polyps.
Results
The baseline, 3-month, and 12-month logarithm of the minimal angle of resolution BCVA was 0.62 ± 0.35, 0.50 ± 0.43, and 0.58 ± 0.48, respectively. Compared to the baseline, patient BCVA was not significantly different at 12 months after diagnosis (p = 0.764). Six eyes (26.1%) gained ≥0.2 logarithm of the minimal angle of resolution BCVA. In cases with subfoveal or juxtafoveal polyps, BCVA values at baseline and at 12 months after diagnosis were 0.66 ± 0.37 and 0.69 ± 0.53, respectively. In cases with extrafoveal polyps, the values were 0.54 ± 0.33 and 0.37 ± 0.31, respectively. Changes in BCVA values were significantly different between the two groups (p = 0.023).
Conclusions
Although anti-VEGF therapy has favorable short-term efficacy for treating PCV with grape-like polyp clusters, long-term visual improvements are generally limited in the majority of afflicted eyes. The presence of subfoveal or juxtafoveal polyps may suggest unfavorable treatment outcomes.
Polypoidal choroidal vasculopathy (PCV) is a disorder of the eyes characterized by branching vascular networks and terminating polypoidal lesions on indocyanine green angiography (ICGA). This condition generally responds well to intravitreal anti-vascular endothelial growth factor (VEGF) therapy [1,2,3,4], photodynamic therapy (PDT) [5,6], or a combination of the two treatments [7]. However, limited knowledge is available regarding anti-VEGF therapy treatment outcomes for PCV presenting with grape-like polyp clusters on ICGA [3,8]. It has been reported that this peculiar PCV generally has unfavorable outcomes when treated with PDT [9]. Additionally, a more recent study shows worse visual outcomes after anti-VEGF therapy in eyes with PCV with grape-like polyp clusters than in eyes with PCV without clusters [3]. That particular study, however, did not include a detailed outcome evaluation in eyes with polyp clusters. To date, only one study has separately analyzed the treatment outcomes of anti-VEGF therapy for eyes with this condition [8].
The present study evaluates 12-month visual and anatomical outcomes of anti-VEGF therapy for eyes newly diagnosed with PCV with grape-like polyp clusters.
This retrospective, observational case series was performed at a single center. All study conduct adhered to the tenets of the Declaration of Helsinki, and the study protocol was approved by the institutional review board.
Eyes with PCV with grape-like polyp clusters that were treated with anti-VEGF monotherapy for 12 months were included. The results of fundus photographs and ICGA were reviewed for patients who were initially diagnosed with PCV between January 2010 and March 2013 at Kim's Eye Hospital. A cluster of grape-like polyps was defined based on some modification of criteria in a previous study [9]; including (1) a hyperfluorescence corresponding to an aneurysmal dilation on late-phase ICGA; (2) appearance of curvilinear, tortuous, or looped vessels, which first branch into smaller vessels before sprouting into numerous polyps of varying size, resulting in a grape-like appearance in the early ICGA phase (Fig. 1A and 1B); and (3) a maximum greatest linear dimension of the polyp cluster of ≥1,000 µm on ICGA. The criterion of "large reddish-orange aneurysmal dilation with the longest axis of 1,500 µm or longer on fundus photograph," which was used in a previous study [9], was not used in the current study. The decision to exclude this criterion was made because, in some eyes, definite reddish-orange aneurysmal dilation is not easily visible on fundus photographs, despite definite ICGA findings. Instead, we used a novel criterion, as described above (criterion 3). Two examiners (JHK and YSC) jointly reviewed ICGA results to determine subject eligibility.
To be included in this study, all subjects were required to undergo a comprehensive ophthalmologic examination, including measurement of best-corrected visual acuity (BCVA), 90-diopter lens slit lamp biomicroscopy, fundus photography, fluorescein angiography, and ICGA with a confocal laser-scanning system (HRA-2; Heidelberg Engineering, Dossenheim, Germany). Horizontal and vertical cross-hair scans centered at the center of the fovea were also performed using spectral domain optical coherence tomography (OCT; Spectral OCT/SLO, OTI Ophthalmic Technologies, Miami, FL, USA). Exclusion criteria included a follow-up period shorter than 12 months, symptom duration greater than 6 months, severe media opacity, end-stage age-related macular degeneration (central geographic atrophy and/or disciform scarring), previous intraocular surgery (except cataract surgery), macroaneurysms, proliferative diabetic retinopathy, central retinal vascular occlusion, and any other retinal disorder that may influence macular microstructure and/or function. Eyes with a submacular hemorrhage of ≥3 disc diameters and involving the fovea at the time of diagnosis were also excluded.
All included eyes were treated with three consecutive monthly intravitreal ranibizumab injections as an initial treatment. Subsequently, patients were examined every 1 to 3 months, as determined by the treating physician. In general, retreatment with an anti-VEGF agent (either ranibizumab or bevacizumab) was performed when intraretinal or subretinal fluid or retinal or subretinal hemorrhages developed with an accompanying increase in macular thickness.
Because volume scans were not routinely performed for every patient, central foveal thickness (CFT) was used for analysis. The CFT was defined as the distance between the internal limiting membrane and Bruch's membrane at the fovea. The CFT was manually measured using the built-in calipers of the OCT software program. The greatest linear dimension of the entire PCV lesion on ICGA was also measured using the built-in calipers of the ICGA software program.
In all included eyes, BCVA and CFT values were compared between baseline measurements and measurements taken at 3 and 12 months after initial diagnosis. Included eyes were divided into two groups according to the location of polyp clusters. Eyes exhibiting subfoveal or juxtafoveal (<200 µm from the fovea) polyps were included in the subfoveal/juxtafoveal polyp group, whereas the remaining eyes were included in the extrafoveal polyp group. The greatest linear dimension of the PCV lesion, the number of anti-VEGF injections during the 12-month follow-up period, the proportion of eyes exhibiting subretinal fluids after three ranibizumab injections, and changes in BCVA and CFT values during the follow-up period were compared between the two groups.
Data are presented as mean ± standard deviations where applicable. Statistical analyses were performed with a commercially available software package (SPSS ver. 12.0; SPSS Inc., Chicago, IL, USA). Differences in values at various time points were tested for statistical significance using a repeated-measures analysis of variance with the Bonferroni correction. Comparison between the two different groups was performed using Mann-Whitney U-tests or Fisher's exact tests. A p-value less than 0.05 was considered statistically significant for all tests.
From the 23 patients, 20 eyes satisfied all eligibility criteria and were included in analyses (Table 1). Fourteen patients (60.9%) were male and 9 patients (39.1%) were female, and the mean patient age was 65.8 ± 6.8 years. The greatest PCV lesion linear dimension averaged 2,401.6 ± 673.2 µm. During the 12-month follow-up period, patients received an average of 4.5 ± 1.4 anti-VEGF injections (3.8 ± 1.5 ranibizumab injections and 0.6 ± 1.1 bevacizumab injections). Subretinal fluid was present after the initial three ranibizumab injections in seven of the 23 eyes (30.4%). A submacular hemorrhage developed in three eyes (13.0%) during the 12-month follow-up period.
Patient BCVA values at baseline, at 3 months, and at 12 months after diagnosis were 0.62 ± 0.35 (Snellen equivalent = 20 / 83), 0.50 ± 0.43 (20 / 63), and 0.58 ± 0.48 (20 / 76), respectively (Fig. 2A). The BCVA values at 3 months were significantly better than the baseline values (p = 0.010), whereas BCVA values at 12 months were not significantly different from the baseline values (p = 0.764). Six eyes (26.1%) gained two or more lines of vision (≥0.2 logarithm of the minimal angle of resolution BCVA), and five eyes (21.7%) lost two or more lines of vision. The remaining 12 eyes (52.2%) had stable BCVA values. The CFT values at baseline, at 3 months, and at 12 months after diagnosis were 453.9 ± 164.8 µm, 282.9 ± 178.9 µm, and 325.4 ± 189.5 µm, respectively (Fig. 2B). The 3- and 12-month CFT values significantly decreased in comparison to baseline values (p < 0.001 and p = 0.005, respectively).
When divided into two groups according to the location of polyp clusters, 15 eyes were included in the subfoveal/juxtafoveal polyp group (Fig. 3A-3E), whereas the remaining eight eyes were included in the extrafoveal polyp group (Fig. 4A-4E). Table 2 summarizes the results of comparisons between the two groups. There were no significant differences in the greatest PCV linear dimension (subfoveal/juxtafoveal vs. extrafoveal, 2,483.5 ± 663.9 µm vs. 2,247.9 ± 707.9 µm; p = 0.349) or number of anti-VEGF injections (4.7 ± 1.3 vs. 4.1 ± 1.6; p = 0.238) between the two groups. In the subfoveal/juxtafoveal group, subretinal fluid was present after the initial three ranibizumab injections in six eyes (40.0%). The BCVA values at baseline, at 3 months, and at 12 months after diagnosis were 0.66 ± 0.37 (20 / 91), 0.54 ± 0.47 (20 / 69), and 0.69 ± 0.53 (20 / 97), respectively, while the CFT values at baseline, at 3 months, and at 12 months after diagnosis were 423.2 ± 156.7 µm, 273.9 ± 165.7 µm, and 341.9 ± 183.2 µm, respectively. In the extrafoveal group, subretinal fluid was present after the initial three ranibizumab injections in one eye (12.5%). The BCVA values at baseline, at 3 months, and at 12 months after diagnosis were 0.54 ± 0.33 (20 / 69), 0.42 ± 0.37 (20 / 52), and 0.37 ± 0.31 (20 / 46), respectively, while the CFT values at baseline, at 3 months, and at 12 months after diagnosis were 468.5 ± 125.7, 299.9 ± 212.7, and 294.6 ± 210.1 µm, respectively. The proportion of eyes exhibiting subretinal fluids after three ranibizumab injections (p = 0.345) and changes in CFT values during the 12-month follow-up period (p = 0.138) were not significantly different between the two groups. On the other hand, there was a significant difference in BCVA changes across the various time points between the two groups (p = 0.023).
In this study, CFT significantly decreased after anti-VEGF therapy in eyes with PCV with grape-like polyp clusters throughout the 12-month follow-up period. Although BCVA was not significantly improved in all eyes at 12 months after diagnosis, an improvement in BCVA of two lines or greater was observed in 26.1% of eyes, which suggests that anti-VEGF therapy has valid effects in some eyes with PCV with grape-like polyp clusters.
Grape-like polyp clusters are noted in approximately 9% to 13% of all eyes with PCV [3,10]. The work of Uyama et al. [11] reports the natural history of PCV with grape-like polypoidal dilations. That study finds that these lesions are usually active and tend to hemorrhage or leak, resulting in severe visual loss in the eyes of patients with PCV with grape-like polypoidal dilations. A study by Lee et al. [9] reports the results of 2-year PDT treatment for PCV with grape-like polyp clusters. Among the patients in that study, median visual acuity improved from 20 / 63 to 20 / 50 after initial treatment, but visual acuity deteriorated during the 2-year follow-up period. At 1 year, 59% of eyes had a three or greater line decrease in visual acuity from baseline values. The authors hypothesized that grape-like polypoidal lesions have a potent neovascular drive, and that possible negative influences of PDT may accelerate this drive. The authors also suggested the possible benefits of anti-VEGF therapy for this condition. According to their findings, however, the visual outcomes after anti-VEGF therapy in eyes with PCV with grape-like polyp clusters were also unfavorable. Recently, Hikichi et al. [3] compared visual outcomes 12 months after anti-VEGF therapy between PCV eyes with and without grape-like polyp clusters, observing unfavorable outcomes in eyes with the cluster. The authors suggested that a greater incidence of submacular hemorrhage in PCV eyes with polyp clusters was the possible cause of this difference in visual outcomes. However, detailed data about changes in visual acuity and macular thickness in PCV eyes with polyp clusters, which may provide valuable treatment response information, was not reported in that study. In the present study, the number of anti-VEGF injections administered over the 12-month follow-up period (a mean of 4.5 injections) was comparable to the number of anti-VEGF injections (mean, 4.2 injections) reported by Hikichi et al. [3]. The incidence of development of submacular hemorrhage during the 12-month follow-up period was 13.0% in the present study.
Previous studies reporting 12-month treatment outcomes of anti-VEGF therapy for PCV have revealed somewhat discrepant results. Although marked improvement in visual acuity is noted in many studies [1,2,3,6], only minimal changes in visual acuity are reported in other studies [12,13,14]. In the present study, a marked decrease in CFT, accompanied by an improvement in BCVA during the first 3 months of anti-VEGF therapy is noted. This result suggests that anti-VEGF therapy has certain short-term benefits for this condition. In contrast, BCVA values deteriorated among eyes in our study between 3 and 12 months, suggesting limited long-term efficacy. In addition, there were differences in response to treatment according to differing polyp cluster locations in the eyes of patients herein.
Extrafoveal polypoidal lesions are not an infrequent occurrence in PCV [15,16]. Although a recent study shows that anti-VEGF therapy is an effective treatment for extrafoveal exudative age-related macular degeneration [17], the efficacy of this treatment for extrafoveal PCV has not been fully elucidated. In the present study, the response to treatment was relatively better in the extrafoveal group than in the subfoveal/juxtafoveal group. A slight improvement in visual acuity was noted in the extrafoveal group, whereas a slight deterioration in visual acuity was noted in the subfoveal/juxtafoveal group. As a result, there was a marked difference in the degree of change in visual acuity between the two groups. This result may suggest that anti-VEGF monotherapy is a useful treatment option in PCV with extrafoveal polyp clusters. When the polyps are located in the subfoveal region or close to the fovea, however, the beneficial effects of anti-VEGF therapy may be limited, suggesting the need for further studies to investigate the efficacy of other approaches. More specifically, recent studies have reported the resolution of fluid and retinal pigment epithelial detachment after intravitreal aflibercept in some PCVs that were refractory to ranibizumab therapy [18,19]. The efficacy of intravitreal aflibercept in PCV eyes with grape-like polyp clusters merits further investigation.
Recently, Lee and Lee [8] reported 24-month outcomes of anti-VEGF monotherapy in PCV eyes with grape-like polyp clusters. In that study, mean logarithm of the minimal angle of resolution visual acuity values at the time of diagnosis, at 3 months, at 12 months, and at 24 months after diagnosis were 0.61 ± 0.28, 0.50 ± 0.30, 0.42 ± 0.27, and 0.44 ± 0.31, respectively. Values of visual acuity at diagnosis and at 3 months after diagnosis are relatively comparable between this previous study [8] and the present study. However, there is a notable difference in the visual acuity values at 12 months between the two studies. In the present study, the mean visual acuity of PCV eyes with grape-like polyp clusters slightly deteriorated from 3 months to 12 months, whereas continuous improvement in visual acuity was noted throughout the 12-month follow-up period in the previous study [8]. As a result, the visual acuity at 12 months was significantly improved relative to baseline values in the previous study [8], whereas the values at the two time points were not different in the present study. Some possible reasons for this discrepancy are as follows. In the previous study [8], the mean number of anti-VEGF injections was 12.5 ± 2.8 during the 2-year follow-up period. The exact number of injections during the first year is not presented in that study. However, considering previous PCV studies in which the number of injections is relatively greater during the first year than during the second year of therapy [3,20], we postulate that the number of injections during the first year in the study by Lee and Lee [8] is greater than the number of injections during the 12-month follow-up period in the present study. The authors of the previous study suggested that grape-like polypoidal lesions have a potent neovascular drive and characteristics of typical choroidal neovascularization, thereby requiring more frequent injections than ordinary PCVs [8]. We postulate that the lower frequency of injections in our study may have caused under-treatment. The lower injection frequency in the present study can be attributed primarily to our follow-up schedule. It is generally recommended that monthly follow-up examinations, including an OCT examination, be conducted for prompt detection and treatment of exudation recurrence [21], because treatment delays may cause poor visual prognoses [22,23]. This strict follow-up schedule was not used in our retrospective study, however, and as a result, follow-up was performed less frequently. Thus we suspect that our follow-up strategy may have caused the unfavorable visual results. In the study by Lee and Lee [8], rescue PDT was performed for two refractory cases. The 12-month visual acuity values of these two patients were relatively good (20 / 25 and 20 / 32, respectively). PDT is considered to have higher efficacy than anti-VEGF therapy in PCV, particularly in regressing polyps [24], although the issue remains controversial [1]. Several expert groups still recommend PDT, with or without anti-VEGF, as the treatment of choice for PCV [25]. In the present study, all the patients were treated with anti-VEGF monotherapy regardless of their response to the therapy. Thus it is possible that overall treatment outcomes may have been influenced by cases that were less responsive to anti-VEGF monotherapy among the patients herein.
This study has several limitations, including its retrospective design and small sample size. Moreover, retreatment was performed at the discretion of the treating physician rather than according to common t reatment guidelines. Because ICGA was not routinely performed after anti-VEGF therapy among the patients herein, it is not possible to estimate the angiographic changes in polypoidal lesions. For the same reason, development of typical choroidal neovascularization during the follow-up period, as shown in a previous study [9], could not be estimated.
In conclusion, the 12-month efficacy of anti-VEGF therapy for improving visual acuity is generally limited in PCV eyes with grape-like polyp clusters. However, definitive improvement in visual acuity was noted in 26.1% of the eyes included herein, which suggests that anti-VEGF therapy has valid effects in a certain proportion of eyes with this condition. In addition, the treatment is likely more beneficial when the polyps are not located in the subfoveal or juxtafoveal regions. Further studies are required to establish more appropriate treatment strategies for PCV with grape-like polyp clusters.
Notes
This study was presented in part at the Association for Research in Vision and Ophthalmology Meeting at Orlando and Florida, USA, 2014.
Conflicts of interest
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
REFERENCES
1. Oishi A, Kojima H, Mandai M, et al. Comparison of the effect of ranibizumab and verteporfin for polypoidal choroidal vasculopathy: 12-month LAPTOP study results. Am J Ophthalmol 2013;156:644-651.


2. Cheng CK, Peng CH, Chang CK, et al. One-year outcomes of intravitreal bevacizumab (avastin) therapy for polypoidal choroidal vasculopathy. Retina 2011;31:846-856.


3. Hikichi T, Higuchi M, Matsushita T, et al. Results of 2 years of treatment with as-needed ranibizumab reinjection for polypoidal choroidal vasculopathy. Br J Ophthalmol 2013;97:617-621.


4. Lee SY, Kim JG, Joe SG, et al. The therapeutic effects of bevacizumab in patients with polypoidal choroidal vasculopathy. Korean J Ophthalmol 2008;22:92-99.



5. Chan WM, Lam DS, Lai TY, et al. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: one-year results of a prospective case series. Ophthalmology 2004;111:1576-1584.


6. Inoue M, Arakawa A, Yamane S, Kadonosono K. Long-term outcome of intravitreal ranibizumab treatment, compared with photodynamic therapy, in patients with polypoidal choroidal vasculopathy. Eye (Lond) 2013;27:1013-1020.



7. Tomita K, Tsujikawa A, Yamashiro K, et al. Treatment of polypoidal choroidal vasculopathy with photodynamic therapy combined with intravitreal injections of ranibizumab. Am J Ophthalmol 2012;153:68-80.e1.


8. Lee JH, Lee WK. Anti-vascular endothelial growth factor monotherapy for polypoidal choroidal vasculopathy with polyps resembling grape clusters. Graefes Arch Clin Exp Ophthalmol 2016;254:645-651.


9. Lee WK, Kim KS, Kim W, et al. Responses to photodynamic therapy in patients with polypoidal choroidal vasculopathy consisting of polyps resembling grape clusters. Am J Ophthalmol 2012;154:355-365.e1.


10. Sho K, Takahashi K, Yamada H, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol 2003;121:1392-1396.


11. Uyama M, Wada M, Nagai Y, et al. Polypoidal choroidal vasculopathy: natural history. Am J Ophthalmol 2002;133:639-648.


12. Lai TY, Lee GK, Luk FO, Lam DS. Intravitreal ranibizumab with or without photodynamic therapy for the treatment of symptomatic polypoidal choroidal vasculopathy. Retina 2011;31:1581-1588.


13. Tsujikawa A, Ooto S, Yamashiro K, et al. Treatment of polypoidal choroidal vasculopathy by intravitreal injection of bevacizumab. Jpn J Ophthalmol 2010;54:310-319.


14. Wakabayashi T, Gomi F, Sawa M, et al. Intravitreal bevacizumab for exudative branching vascular networks in polypoidal choroidal vasculopathy. Br J Ophthalmol 2012;96:394-399.


15. Gemmy Cheung CM, Yeo I, Li X, et al. Argon laser with and without anti-vascular endothelial growth factor therapy for extrafoveal polypoidal choroidal vasculopathy. Am J Ophthalmol 2013;155:295-304.e1.


16. Hou J, Tao Y, Li XX, Zhao MW. Clinical characteristics of polypoidal choroidal vasculopathy in Chinese patients. Graefes Arch Clin Exp Ophthalmol 2011;249:975-979.


17. Parodi MB, Iacono P, La Spina C, et al. Intravitreal ranibizumab for naive extrafoveal choroidal neovascularization secondary to age-related macular degeneration. Retina 2014;34:2167-2170.


18. Saito M, Kano M, Itagaki K, et al. Switching to intravitreal aflibercept injection for polypoidal choroidal vasculopathy refractory to ranibizumab. Retina 2014;34:2192-2201.


19. Yamashita M, Nishi T, Hasegawa T, Ogata N. Response of serous retinal pigment epithelial detachments to intravitreal aflibercept in polypoidal choroidal vasculopathy refractory to ranibizumab. Clin Ophthalmol 2014;8:343-346.


20. Kang HM, Koh HJ. Long-term visual outcome and prognostic factors after intravitreal ranibizumab injections for polypoidal choroidal vasculopathy. Am J Ophthalmol 2013;156:652-660.


21. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol 2007;143:566-583.


22. Rauch R, Weingessel B, Maca SM, Vecsei-Marlovits PV. Time to first treatment: The significance of early treatment of exudative age-related macular degeneration. Retina 2012;32:1260-1264.


23. Arias L, Armada F, Donate J, et al. Delay in treating age-related macular degeneration in Spain is associated with progressive vision loss. Eye (Lond) 2009;23:326-333.


Fig. 1
Indocyanine green angiography images (A,B) from eyes diagnosed with polypoidal choroidal vasculopathy with grape-like polyp clusters.
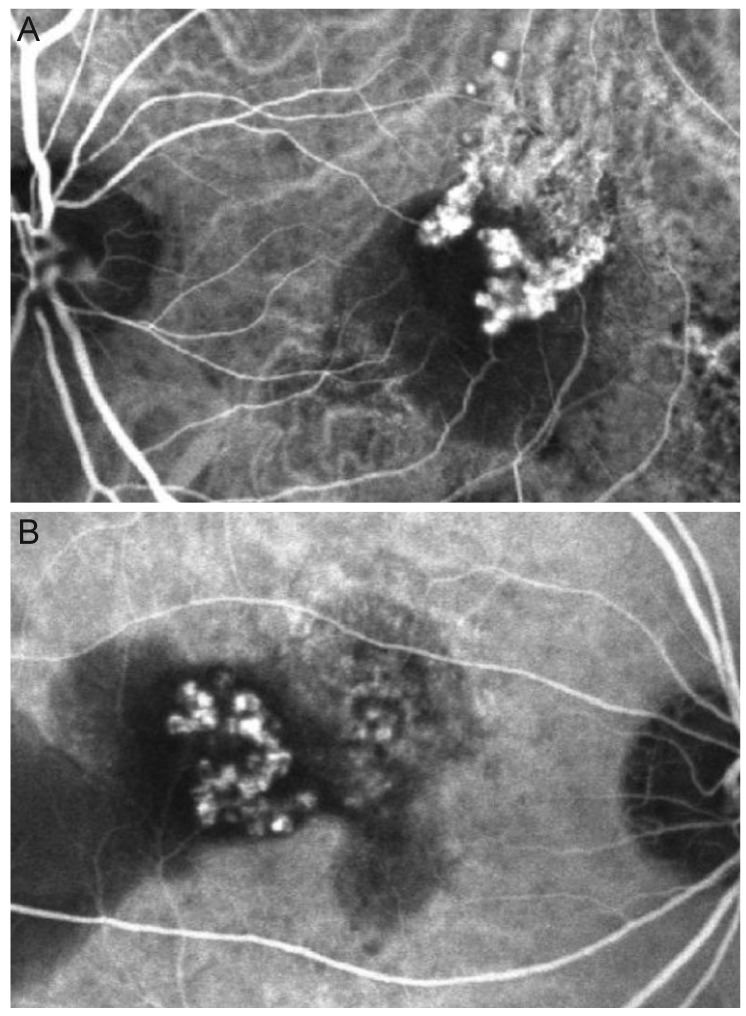
Fig. 2
Changes in the mean logarithm of minimal angle of resolution (logMAR) best-corrected visual acuity (BCVA) (A) and central foveal thickness (B) in eyes diagnosed with polypoidal choroidal vasculopathy with grape-like polyp clusters. Statistical analyses were performed using repeated measures analysis of variances with the Bonferroni correction.

Fig. 3
Fundus photography (A), fluorescein angiography (B), indocyanine green angiography (C), and optical coherence tomography (D,E) images at diagnosis (A-D) and at 12 months (E) from diagnosis in an eye with polypoidal choroidal vasculopathy with grape-like polyp clusters (arrow, C). In this case, the polyps were located at the fovea. Subretinal fluid was observed at 12 months after diagnosis (E).
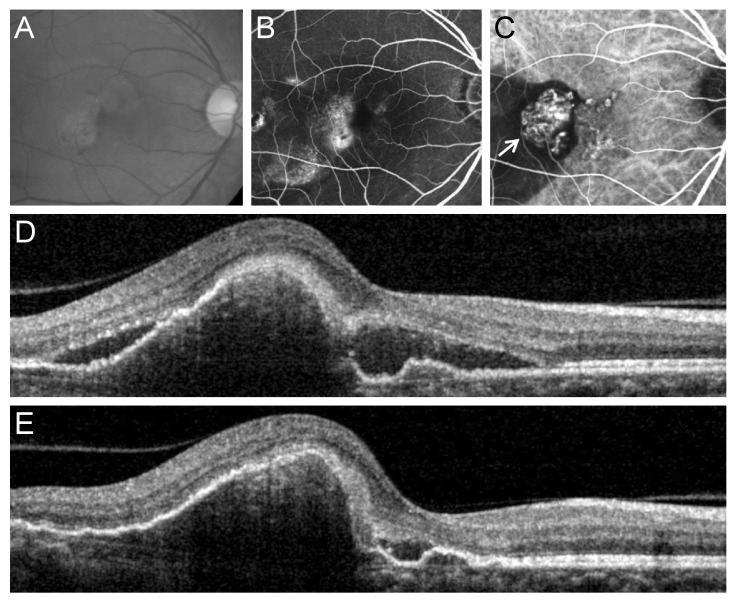
Fig. 4
Fundus photography (A), fluorescein angiography (B), indocyanine green angiography (C), and optical coherence tomography (D,E) images at diagnosis (A-D) and at 12 months (E) from diagnosis in an eye with polypoidal choroidal vasculopathy with grape-like polyp clusters (arrow, C). In this case, the polyps were located outside the fovea. At 12 months after diagnosis (E), complete resolution of the fluid was noted.
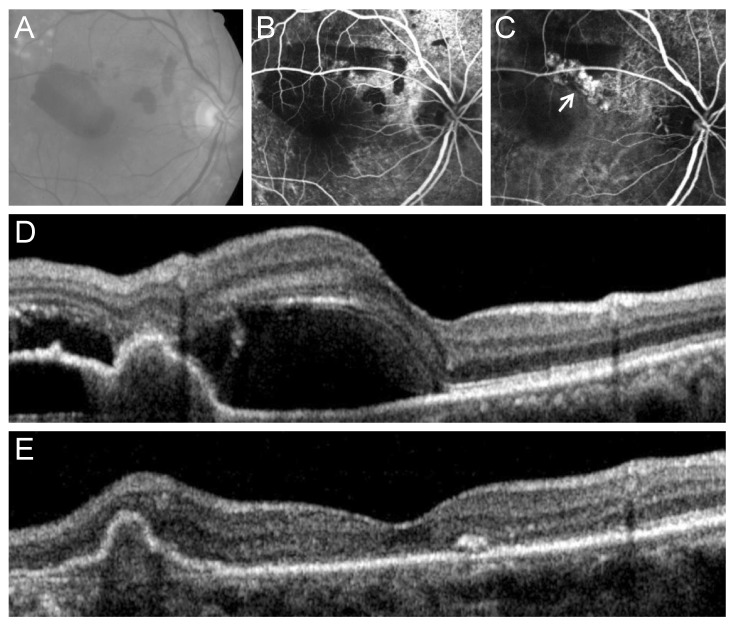
Table 1
Baseline characteristics of eyes with polypoidal choroidal vasculopathy with grape-like polyp clusters
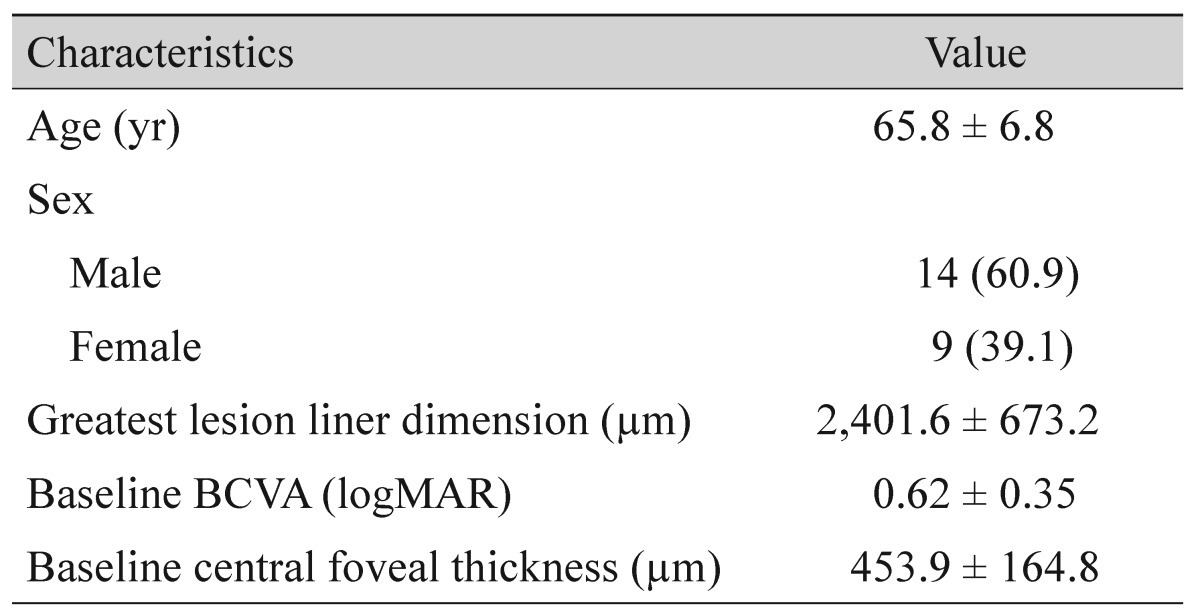
Table 2
Comparison of characteristics between eyes exhibiting subfoveal or juxtafoveal polyps and eyes exhibiting extrafoveal polyps

Values are presented as mean ± standard deviation or number (%).
VEGF = vascular endothelial growth factor; BCVA = best-corrected visual acuity; logMAR = logarithm of the minimal angle of resolution.
*Mann-Whitney U-test; †Fisher's exact test; ‡Positive value indicates improvement in visual acuity, whereas negative value indicates deterioration in visual acuity.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print